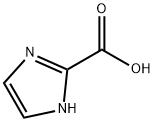
1H-Imidazole-2-carboxylic acid synthesis
- Product Name:1H-Imidazole-2-carboxylic acid
- CAS Number:16042-25-4
- Molecular formula:C4H4N2O2
- Molecular Weight:112.09
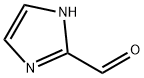
10111-08-7

16042-25-4
General procedure for the synthesis of imidazole-2-carboxylic acid from 2-imidazolecarboxaldehyde: 30% aqueous H2O2 solution (10 g) was slowly added dropwise to stirred aqueous 2-imidazolecarboxaldehyde (2.88 g, 0.030 mol) solution (10 ml). The reaction was continued at room temperature for 72 hours. After completion of the reaction, water was removed by distillation under reduced pressure at room temperature to give a white crystalline solid. The resulting solid was washed with stirred diethyl ether/water (4:1) mixture to remove residual peroxide. Note: Heating may cause decarboxylation to occur. The yield was 97.5%. Melting point (MP) was 156-158 °C. 1H NMR (400 MHz, D2O): δ 7.56 (2H, s, imidazolium ring-H); 13C NMR (400 MHz, D2O): δ 158.86, 141.02, 120.49 ppm. IR (KBr, cm-1): 3392 (m), 3124 (m), 2861 ( m), 1618 (s), 1502 (m), 1462 (m), 1421 (s), 1388 (s), 1322 (m), 1108 (s), 925 (s), 910 (s), 819 (m), 797 (s), 774 (m). Calculated values for elemental analysis (C4H6N2O3, 130.11): C, 36.92%; H, 4.65%; N, 21.53%. Measured values: C, 37.18%; H, 4.94%; N, 21.47%.

10111-08-7
349 suppliers
$6.00/1g

16042-25-4
197 suppliers
$10.00/250mg
Yield:16042-25-4 97.5%
Reaction Conditions:
with dihydrogen peroxide in water at 20; for 72 h;
Steps:
2.2.1 Im2COOH (1b)
An aqueous 30% H2O2 solution (10g) was added dropwise to a stirred solution of imidazole-2-carboxaldehyde (2.88g 0.030mol) in water (10ml). The reaction was allowed to proceed at room temperature for 72h, following which the water was removed in vacuo at room temperature to afford a white crystalline solid. This solid was washed with a stirred mixture of diethylether/water (4:1) to remove the excess peroxide. Note: heating causes decarboxylation. Yield: 97.5%. Mp=156-158°C. δH (400MHz, D2O): 7.56 (2H, s, Im-H); δC (400MHz, D2O): 158.86, 141.02, 120.49ppm. IR ν (KBr): 3392(m), 3124(m), 2861(m), 1618(s), 1502(m), 1462(m), 1421(s), 1388(s), 1322(m), 1108(s), 925(s), 910(s), 819(m), 797(s), 774(m) cm-1. Anal. Calc. for C4H6N2O3 (130.11); C, 36.92; H, 4.65; N, 21.53%. Found: C, 37.18; H, 4.94; N, 21.47%.
References:
Gundhla, Isaac Z.;Walmsley, Ryan S.;Ugirinema, Vital;Mnonopi, Nandipha O.;Hosten, Eric;Betz, Richard;Frost, Carminita L.;Tshentu, Zenixole R. [Journal of Inorganic Biochemistry,2015,vol. 145,p. 11 - 18]
33543-78-1
216 suppliers
$15.00/1 / G

16042-25-4
197 suppliers
$10.00/250mg
19213-72-0
65 suppliers
$21.00/1g

16042-25-4
197 suppliers
$10.00/250mg
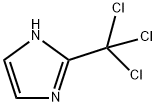
163769-73-1
9 suppliers
inquiry

16042-25-4
197 suppliers
$10.00/250mg
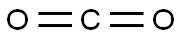
124-38-9
132 suppliers
$175.00/23402

61278-81-7
41 suppliers
$233.00/10ml

16042-25-4
197 suppliers
$10.00/250mg