
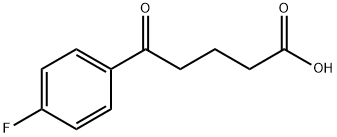
(4S)-3-[5-(4-Fluorophenyl)-1,5-dioxopenyl]-4-phenyl-2-oxazolidinone synthesis
- Product Name:(4S)-3-[5-(4-Fluorophenyl)-1,5-dioxopenyl]-4-phenyl-2-oxazolidinone
- CAS Number:189028-93-1
- Molecular formula:C20H18FNO4
- Molecular Weight:355.36

99395-88-7
149437-76-3
![(4S)-3-[5-(4-Fluorophenyl)-1,5-dioxopenyl]-4-phenyl-2-oxazolidinone](/CAS/GIF/189028-93-1.gif)
189028-93-1
Example 1: Preparation of ezetimibe; (1-1) Preparation of (4S)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone (Eq. 7); A reaction mixture was prepared by dissolving 200 g of 5-(4-fluorophenyl)-5-oxopentanoic acid (Eq. 8), 160 g of (S)-4-phenyl-oxazolidin-2-one (Eq. 9), and 11.6 g of 4- dimethylamino pyridine were dissolved in 600 mL of dichloromethane to prepare the reaction mixture. A solution prepared by dissolving 157 g of N,N'-dicyclohexylcarbimide in 200 mL of dichloromethane was added to the reaction mixture and stirred for 2 hours. Upon completion of the reaction, the resulting reaction mixture was filtered to remove the by-products. The filtrate was washed sequentially with 1 L of 6N HCl, 1 L of water and 1 L of saturated sodium chloride solution, dried with anhydrous magnesium sulfate, filtered and the solvent was removed by distillation under reduced pressure. The residue was dissolved in 2 L of methanol by heating and cooled to induce crystallization. 2L of water was added and stirred for 30 minutes. The solid was separated by filtration to give 289 g of (4S)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone as a white solid (yield: 86%).1H NMR (300 MHz, CDCl3): δ 7.92 (2H, m), 7.35-7.13 (5H, m), 7.04 (2H, m). 5.43 (1H, q), 4.75 (1H, t), 4.22 (1H, q), 3.05-2.93 (4H, m), 2.03 (2H, m).

99395-88-7
506 suppliers
$5.00/5g

149437-76-3
383 suppliers
$8.00/10g
![(4S)-3-[5-(4-Fluorophenyl)-1,5-dioxopenyl]-4-phenyl-2-oxazolidinone](/CAS/GIF/189028-93-1.gif)
189028-93-1
371 suppliers
$6.00/1g
Yield:189028-93-1 86%
Reaction Conditions:
with dmap;dicyclohexyl-carbodiimide in dichloromethane
Steps:
1.1
Example 1: Preparation of ezetimibe; (1-1) Preparation of 3-[5-(fluorophenyl)-l,5-dioxapentyl]-4(S)- phenyl-2-oxazolidinone (Formula 7); 200 g of 5-(4-fluorophenyl)-5-oxopentanoic acid of formula 8, 16O g of (S)-4-phenyloxazolidine-2-one of formula 9, and 11.6 g of 4-dimethylaminopyridine were dissolved in 600 m£ of dichloromethane to prepare a reaction mixture. A solution which was prepared by dissolving 157 g of N,N'-dicyclohexylcarboimide in 200 ml of dichloromethane was added to the reaction mixture and stirred for 2 hours. After completion of the reaction, the resulting reaction mixture was filtered to remove by-products. The filtrate thus obtained was washed successively with 1 I of 6N HCl, 1 € of water, and 1 € of saturated sodium chloride, dried over anhydrous magnesium sulfate, filtered, and distilled under a reduced pressure to remove the solvent. The residue thus obtained was dissolved in 2 I of methanol by heating and cooled to induce crystallization. 2 £ of water was added thereto and stirred for 30 min. The solid thus obtained was isolated by filtering to obtain 289 g of the title compound as a white solid (yield: 86%).1H NMR(300MHz, CDCl3) : δ 7.92 (2H, M), 7.35-7.13 (5H, m), 7.04 (2H, m), 5.43 (IH, q), 4.75(1H, t), 4.22 (IH, q), 3.05-2.93 (4H, m), 2.03 (2H, m)
References:
HANMI PHARM. CO., LTD.;KIM, Gi Jeong;KIM, Choong Hahn;CHANG, Ji Yeon;KIM, Nam Du;CHANG, Young Kil;LEE, Gwan Sun WO2010/71358, 2010, A2 Location in patent:Page/Page column 14-15

3282-30-2
380 suppliers
$18.00/100ml

149437-76-3
383 suppliers
$8.00/10g

186343-35-1
2 suppliers
inquiry
![(4S)-3-[5-(4-Fluorophenyl)-1,5-dioxopenyl]-4-phenyl-2-oxazolidinone](/CAS/GIF/189028-93-1.gif)
189028-93-1
371 suppliers
$6.00/1g

1056188-47-6
14 suppliers
inquiry

99395-88-7
506 suppliers
$5.00/5g
![(4S)-3-[5-(4-Fluorophenyl)-1,5-dioxopenyl]-4-phenyl-2-oxazolidinone](/CAS/GIF/189028-93-1.gif)
189028-93-1
371 suppliers
$6.00/1g
![2-Oxazolidinone, 3-[5-(4-fluorophenyl)-5-hydroxy-1-oxopentyl]-4-phenyl-, (4S)-](/CAS/GIF/1246853-48-4.gif)
1246853-48-4
0 suppliers
inquiry
![(4S)-3-[5-(4-Fluorophenyl)-1,5-dioxopenyl]-4-phenyl-2-oxazolidinone](/CAS/GIF/189028-93-1.gif)
189028-93-1
371 suppliers
$6.00/1g
![Benzene, 1-fluoro-4-[1-[(trimethylsilyl)oxy]ethenyl]-](/CAS/20200515/GIF/58518-77-7.gif)
