
N-TERT-BUTOXYCARBONYL-4-(4-TOLUENESULFONYLOXYMETHYL)PIPERIDINE synthesis
- Product Name:N-TERT-BUTOXYCARBONYL-4-(4-TOLUENESULFONYLOXYMETHYL)PIPERIDINE
- CAS Number:166815-96-9
- Molecular formula:C18H27NO5S
- Molecular Weight:369.48

98-59-9

123855-51-6

166815-96-9
Example 1A Synthesis of tert-butyl 4-((tolylenesulfonyloxy)methyl)piperidine-1-carboxylate: N-BOC-4-piperidine methanol (10 g, 46.5 mmol) and triethylamine (5.64 g, 55.8 mmol) were dissolved in dichloromethane (100 mL) under nitrogen protection and cooled to 0°C. Subsequently, p-toluenesulfonyl chloride (9.73 g, 51.2 mmol) and 4-dimethylaminopyridine (1.13 g, 9.3 mmol) were slowly added to the solution. The reaction mixture was stirred at 17°C for 2 hours. After completion of the reaction, the reaction was quenched by the addition of water (100 mL). The aqueous layer was extracted with dichloromethane (100 mL x 2). The organic layers were combined, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give the crude product. Purification by column chromatography afforded 1-N-Boc-4-(4-methylbenzenesulfonyl oxymethyl)piperidine (17 g, 99% yield) as a white solid.LCMS (ESI) m/z: 370 (M + 1).
280-57-9
589 suppliers
$5.00/5g
107-83-5
242 suppliers
$10.00/1g

98-59-9
627 suppliers
$9.00/5g

123855-51-6
415 suppliers
$6.00/5g

166815-96-9
166 suppliers
$7.00/250mg
Yield: 85%
Reaction Conditions:
in diethyl ether;tert-butyl methyl ether
Steps:
9.c Preparation of Compound 9 in Table 2
c) 1,4-Diazabicyclo[2.2.2]octane (42.4 g, 0.378 mol) was added to a solution of 4-hydroxymethyl-1-tert-butyloxycarbonylpiperidine (52.5 g, 0.244 mol) in tert-butyl methyl ether (525 ml) and the reaction stirred at ambient temperature for 15 minutes. The reaction was cooled to 5° C. and a solution of 4-toluenesulphonyl chloride (62.8 g, 0.33 mmol) in tert-butyl methyl ether (525 ml) was added dropwise over 2 hours while maintaining the temperature at 0° C. The reaction was stirred at ambient temperature for 1 hour, isohexane was added and the resultant precipitate was collected by suction filtration. Solvent evaporation in vacuo afforded a solid which was dissolved in diethyl ether (250 ml) and washed successively with 0.5N aqueous hydrochloric acid (2*500 ml), water, saturated sodium hydrogen carbonate and brine. Solvent evaporation and drying in vacuo yielded 4-(4-methylphenylsulphonyloxy-methyl)-1-tert-butyloxy-carbonylpiperidine (76.7 g, 85% yield) as a white solid: 1H NMR (CDCl3): 7.80 (d, 2H), 7.35 (d, 2H), 4.00-4.20 (s, 2H), 3.85 (d, 1H), 2.55-2.75 (m, 2H), 2.45 (s, 3H), 1.75-1.90 (m, 2H), 1.65 (d, 2H), 1.45 (s, 9H), 1.00-1.20 (m, 2H): MS (+ve ESI): 392 (M+Na)+.
References:
AstraZeneca AB US7235559, 2007, B1

98-59-9
627 suppliers
$9.00/5g

123855-51-6
415 suppliers
$6.00/5g

166815-96-9
166 suppliers
$7.00/250mg
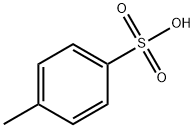
104-15-4
760 suppliers
$133.00/500g

123855-51-6
415 suppliers
$6.00/5g

166815-96-9
166 suppliers
$7.00/250mg
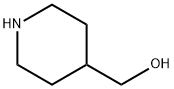
6457-49-4
367 suppliers
$18.00/25g

166815-96-9
166 suppliers
$7.00/250mg

24424-99-5
865 suppliers
$13.50/25G

166815-96-9
166 suppliers
$7.00/250mg