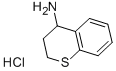
(3,4-dihydro-2H-1-benzothiopyran-4-yl)ammonium chloride synthesis
- Product Name:(3,4-dihydro-2H-1-benzothiopyran-4-yl)ammonium chloride
- CAS Number:15857-70-2
- Molecular formula:C9H12ClNS
- Molecular Weight:201.7163
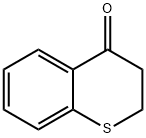
3528-17-4
73 suppliers
$60.00/50mg

593-56-6
630 suppliers
$8.00/100dtn(0.1g)

15857-70-2
18 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 2,3-dihydro-4H-[1]benzothiopyran-4-one;N-methoxylamine hydrochloridewith pyridine at 20; for 4 h;
Stage #2: with borane-THF in tetrahydrofuran at 0 - 90;
Stage #3: with hydrogenchloride in tetrahydrofuran;water at 90;Cooling with ice;
Steps:
27.1
(Step 1) 2,3-Dihydro-4H-thiochromen-4-one (13.0 g) and o-methylhydroxylamine hydrochloride (7.93 g) were stirred in pyridine (30 ml) at room temperature for 4 hr. The reaction solution was poured into water, and the mixture was extracted with ethyl acetate. The extract was washed with 1N hydrochloric acid and saturated brine, dried over magnesium sulfate and filtered. The solvent was evaporated under reduced pressure. To a solution (150 ml) of the obtained residue in tetrahydrofuran was added tetrahydrofuran-borane (200 ml, 1M tetrahydrofuran solution) at 0°C. The reaction mixture was stirred at 90°C for 3 hr, and quenched with ice, and 1N hydrochloric acid (300 ml) was added. The mixture was stirred at 90°C for 2 hr, and ethyl acetate was added thereto. The separated aqueous layer was basified with 8N sodium hydroxide solution, and extracted with ethyl acetate. The extract was washed with saturated brine, dried over magnesium sulfate and filtered. The solvent was evaporated under reduced pressure. The obtained residue was dissolved in methanol, 4N hydrogen chloride-ethyl acetate solution (20 ml) was added, and the obtained precipitate was collected by filtration, and washed with ethyl acetate to give 3,4-dihydro-2H-thiochromen-4-amine hydrochloride (6.16 g). 1H NMR (300 M Hz, DMSO-d6) δ ppm 2.12-2.27 (1H, m) 2.36-2.48 (1H, m) 2.96-3.29 (2H, m) 4.52 (1H, d, J=3.41 Hz) 7.09-7.31 (3H, m) 7.53 (1H, d, J=7.95 Hz) 8.66 (3H, s).
References:
EP2269989,2011,A1 Location in patent:Page/Page column 46-47