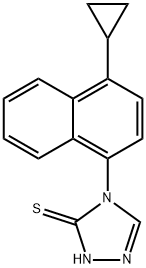
4-(4-cyclopropylnaphthalen-1-yl)-1H-1,2,4-triazole-5(4H)-thione synthesis
- Product Name:4-(4-cyclopropylnaphthalen-1-yl)-1H-1,2,4-triazole-5(4H)-thione
- CAS Number:1533519-84-4
- Molecular formula:C15H13N3S
- Molecular Weight:267.35
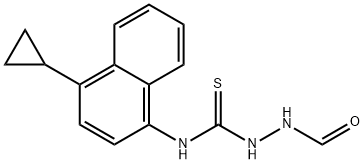
1533519-86-6

1533519-84-4
Example 5. Preparation of 4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazole-3-thiols To a round-bottomed flask was added N-(4-cyclopropyl-1-naphthalenyl)-2-formylhydrazine thiocarboxamide (64.0 g, 225 mmol), N,N-dimethylformamide (300 mL), and 1 mol/L aqueous sodium hydroxide solution (225 mL) to form a mixture. After stirring, the mixture was heated to 30 °C and kept for 16 hours. After the reaction was completed, the mixture was cooled to room temperature (about 20°C). Subsequently, 1 mol/L hydrochloric acid (about 200 mL) was slowly added to the mixture until the pH of the mixture was about 6.0, and then water (about 500 mL) was added. The mixture was stirred for 15 minutes and then filtered. The filter cake was washed with water and the product was dried under vacuum at 50 °C for 5 h to give 4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazole-3-thiol as a gray-green solid, 54.0 g in 90% yield. LC-MS: m/z (ESI): 268 (M + H)+. 1H NMR (400 MHz, acetone-d6): δ 8.57 (d, J = 8.0 Hz, 1H), 8.37 (s, 1H), 7.67 (m, 1H), 7.60 (m, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.48 (d, J = 8.0 Hz, 1H), 7.43 (d, J = 8.0 Hz, 1H), 2.50 (m, 1H), 1.16 (m, 2H), 0.84 (m, 2H).

1533519-86-6
42 suppliers
inquiry

1533519-84-4
179 suppliers
$12.00/250mg
Yield: 90%
Reaction Conditions:
with water;sodium hydroxide in N,N-dimethyl-formamide at 30; for 16 h;Solvent;Temperature;Reagent/catalyst;
Steps:
5 Example 5. Preparation of 4-(4-cyclopropylnaphthalen-l-yl)-4H-l,2,4-triazole-3-thiol
Example 5. Preparation of 4-(4-cyclopropylnaphthalen-l-yl)-4H-l,2,4-triazole-3-thiol [070] To a round bottom flask added 4-(4-cyclopropyl-naphthalen-l-yl) -1-formylamino-thiourea (64.0 g, 225 mmol), DMF (300 mL) and 1 mol/L aqueous sodium hydroxide solution (225 mL) to form a mixture, the mixture was heated to about 30°C after stirred for about 16 hours, the mixture was cooled to room temperature (about 20°C). 1 mol/L hydrochloric acid (about 200 mL) was added into the mixture until the pH value of the mixture was about 6.0, then water (about 500 mL) was added. The mixture was stirred for about 15 minutes and filtered. The filter cake was washed with water, and the product was dried in vacuum at about 50°C for about 5 hours to obtain gray-green solid 4-(4-cyclopropylnaphthalen-l-yl)-4H-l,2,4-triazole-3 -thiol, 54.0 g, yield 90%. LC-Ms: m/z (ESI): 268(M+H)+, 1H MR (400 MHz, acetone-d6): δ 8.57 (d, J=8.0 Hz, 1 H), 8.37 (s, 1 H), 7.67 (m, 1 H), 7.60(m, 1 H), 7.5 l(d, J=8.0 Hz, 1 H), 7.48 (d, J=8.0 Hz, 1 H), 7.43 (d, J=8.0 Hz, 1 H), 2.50 (m, 1 H), 1.16 (m, 2 H), 0.84 (m, 2 H).
References:
SUNSHINE LAKE PHARMA CO., LTD.;CHEN, Weiqiang;LUO, Jian;LIU, Lixue;FAN, Yuping WO2014/198241, 2014, A1 Location in patent:Paragraph 069-070
878671-94-4
84 suppliers
$45.00/250mg

1533519-84-4
179 suppliers
$12.00/250mg

90-11-9
536 suppliers
$5.00/10g

1533519-84-4
179 suppliers
$12.00/250mg

25033-19-6
147 suppliers
$34.00/1g

1533519-84-4
179 suppliers
$12.00/250mg

878671-93-3
31 suppliers
inquiry

1533519-84-4
179 suppliers
$12.00/250mg