
(R,E)-ethyl 5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoate synthesis
- Product Name:(R,E)-ethyl 5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoate
- CAS Number:149709-59-1
- Molecular formula:C25H31NO4
- Molecular Weight:409.52

149818-98-4

21382-82-1
![(R,E)-ethyl 5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoate](/CAS/20150408/GIF/149709-59-1.gif)
149709-59-1
Step 5: 23.3 g of the product of Step 4 ((R,E)-ethyl 5-([1,1'-biphenylyl]-4-yl)-4-((tert-butoxycarbonyl)amino)-2-methylpent-2-enoate, 65.6 mmol, 1.0 eq.) was dissolved in 300 mL of dichloromethane (DCM) and cooled under a nitrogen (N2) atmosphere to -70° C. At this temperature, dropwise 109 mL of diisobutylaluminum hydride (DIBAL-H, 1.5 M toluene solution, 164 mmol, 2.5 eq.) was added. After stirring for 15 minutes, thin layer chromatography (TLC) analysis showed that there was still raw material residue. Subsequently, an additional 15 mL of DIBAL-H (1.5 M toluene solution) was added dropwise and stirring was continued for 5 minutes at -70°C. TLC showed that the reaction was complete. The reaction solution was slowly poured into 450 mL of 1 M aqueous hydrochloric acid (HCl), keeping the temperature below 0°C. After stirring for a few minutes, the organic layer was separated and the aqueous phase was extracted with DCM (100 mL × 2). The combined organic layers were washed sequentially with aqueous sodium bicarbonate (NaHCO3) (150 mL) and water (100 mL) and dried over anhydrous sodium sulfate (Na2SO4). After filtration, 19.0 g of ethyl 2-(triphenylphosphoranylidene)propionate was added to the solution, keeping the temperature below 5°C. The resulting solution was stirred at 25°C for 18 h. TLC showed that the reaction was complete. The reaction solution was concentrated, purified by silica gel column chromatography (petroleum ether/ethyl acetate = 4/1) and then crystallized from petroleum ether/ethyl acetate (80 mL/20 mL) to afford 17.0 g of ethyl (4R)-5-[1,1'-biphenyl]-4-yl-4-[[tert-butoxycarbonyl]amino]-2-methyl-2-pentenoate (chemical purity > 99.5%) as a white solid. Total yield in two steps: 89.1%.

54356-04-6
2 suppliers
inquiry
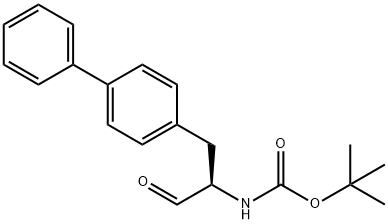
149709-58-0
50 suppliers
inquiry
![(R,E)-ethyl 5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoate](/CAS/20150408/GIF/149709-59-1.gif)
149709-59-1
95 suppliers
$11.00/100mg
Yield:149709-59-1 79.08%
Reaction Conditions:
with Na2S2O3 in dichloromethane;lithium hydroxide monohydrate at 0 - 10;Flow reactor;Large scale;
Steps:
1.4; 1.6-1.8; 2.4; 2.6-2.8; 3.4; 3.6-3.8 Example 1
(1) Preparation of material A solution: Compound II (100 Kg, 305.42 mol, 1.0 eq.) and TEMPO (9.54 Kg, 61.06 mol, 0.20 eq.) were dissolved in dichloromethane (680 Kg); (2) Preparation of material B solution: sodium bicarbonate (38.48 Kg, 458.12 mol, 1.5 eq.) and sodium bromide (1.88 Kg, 18.27 mol, 0.06 eq.) were dissolved in water (600 Kg); (3) Material C solution: sodium hypochlorite aqueous solution, the measured content is: 9.6%; (4) Preparation of material D solution: In the reactor, add water (200 Kg), dichloromethane (132.5 Kg), ethoxyformylethylenetriphenylphosphine (110 Kg, 305.42 mol, 1.0 eq. ) and sodium thiosulfate (15.16 Kg, 61.08 mol, 0.2 eq.), stirred and cooled to 05 for use; (5) Mix the material A solution and the material B solution in the micro-mixer 1 first, the residence time is 4-6 s, and then mix with the material C solution in the micro-mixer 2, the residence time is 3-5 s, and then Enter the microreactor for reaction, the residence time is 20-25 s, the temperature of the micro-mixer is 0-20 ; the temperature of the micro-reactor is 0-30 ; (6) The reaction solution obtained in step (5) is directly flowed into the reaction kettle containing the material D solution in step (4), and the reaction is stirred at a reaction temperature of 0 to 10 °C; (7) The reaction solution of step (6) is left to stand for stratification, the aqueous phase is extracted with dichloromethane, and the organic phases are combined and concentrated to obtain Sacubitril intermediate (IV); (8) Refining: the Sacubitril intermediate (IV) obtained in step (7) is heated and dissolved in ethanol, the system is in a light yellow and clear state, and water is added dropwise to the system while maintaining the temperature at 68-73°C, and there is solid Precipitation, turn off heating, gradient cooling, centrifugation, to obtain 138 Kg of wet product, and to obtain 98.92 Kg of refined intermediate (IV) after drying, yield: 79.08%, purity: 99.8998%.
References:
CN113754565,2021,A Location in patent:Paragraph 0030-0032; 0034

149709-58-0
50 suppliers
inquiry

5717-37-3
458 suppliers
$6.00/5g
![(R,E)-ethyl 5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoate](/CAS/20150408/GIF/149709-59-1.gif)
149709-59-1
95 suppliers
$11.00/100mg

149818-98-4
25 suppliers
inquiry

5717-37-3
458 suppliers
$6.00/5g
![(R,E)-ethyl 5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoate](/CAS/20150408/GIF/149709-59-1.gif)
149709-59-1
95 suppliers
$11.00/100mg
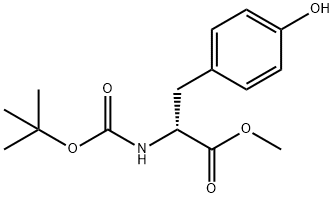
76757-90-9
157 suppliers
$14.00/1G
![(R,E)-ethyl 5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoate](/CAS/20150408/GIF/149709-59-1.gif)
149709-59-1
95 suppliers
$11.00/100mg
![D-Tyrosine, N-[(1,1-dimethylethoxy)carbonyl]-O-[(trifluoromethyl)sulfonyl]-, methyl ester](/CAS/20180703/GIF/149709-56-8.gif)
149709-56-8
16 suppliers
inquiry
![(R,E)-ethyl 5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoate](/CAS/20150408/GIF/149709-59-1.gif)
149709-59-1
95 suppliers
$11.00/100mg