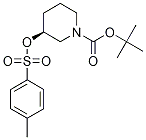
(S)-3-(Toluene-4-sulfonyloxy)-piperidine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:(S)-3-(Toluene-4-sulfonyloxy)-piperidine-1-carboxylic acid tert-butyl ester
- CAS Number:1353993-49-3
- Molecular formula:C17H25NO5S
- Molecular Weight:355.4491

98-59-9
622 suppliers
$9.00/5g
143900-44-1
561 suppliers
$5.00/1g

1353993-49-3
10 suppliers
$350.00/1g
Yield:1353993-49-3 93%
Reaction Conditions:
with dmap;triethylamine in dichloromethane at 20;Cooling with ice;
Steps:
1.G
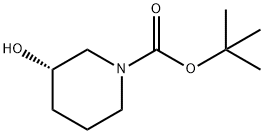
[0137] Tert-butyl (S)-3-(tosyloxy)piperidine-1-carboxylate: To 50 mL of CH2Cl2 in an ice bath was added tert-butyl (S)-3-hydroxypiperidine-1-carboxylate (3 g, 1.0 eq) followed by slow addition of 4-methylbenzenesulfonyl chloride (3.4 g, 1.2 eq), N,N-dimethylpyridin-4-amine (0.1 g, 10%) and triethylamine (3 g, 2.0 eq).
After completion of addition, the reaction mixture was warmed up to room temperature and stirred overnight.
The organic phase was washed with water and brine, dried over anhydrous Na2SO4, and isolated through silica gel column chromatography to provide the product as a white solid (4.9 g, 93%).
1H NMR (400 MHz, CDCl3) δ7.80 (d, J= 8.0 Hz, 2H), 7.34 (d, J= 8.0 Hz, 2H), 4.46 (brs, 1H), 3.54-3.58 (m, 1H), 3.31-3.40 (m, 3H), 2.45 (s, 3H), 1.80-1.88 (m, 1H), 1.65-1.79 (m, 2H), 1.47-1.52 (m, 1H), 1.43 (s, 9H).
References:
EP3141546,2017,A1 Location in patent:Paragraph 0137; 0138