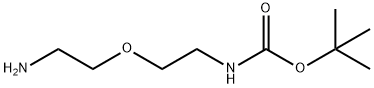
Carbamic acid, [2-(2-aminoethoxy)ethyl]-, 1,1-dimethylethyl ester (9CI) synthesis
- Product Name:Carbamic acid, [2-(2-aminoethoxy)ethyl]-, 1,1-dimethylethyl ester (9CI)
- CAS Number:127828-22-2
- Molecular formula:C9H20N2O3
- Molecular Weight:204.27
![Carbamic acid, N-[2-(2-azidoethoxy)ethyl]-, 1,1-dimethylethyl ester](/CAS/20200331/GIF/176220-30-7.gif)
176220-30-7
![Carbamic acid, [2-(2-aminoethoxy)ethyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/127828-22-2.gif)
127828-22-2
Step 4: Synthesis of tert-butyl [2-(2-aminoethoxy)ethyl]carbamate (58) Under argon protection, tert-butyl (2-(2-azidoethoxy)ethyl)carbamate (57, 5.0 g, 0.0217 mol) was dissolved in methanol (100 mL) and palladium/carbon catalyst (2.5 g, 10 mL) was added. The reaction mixture was stirred overnight at room temperature under hydrogen (50 psi) atmosphere. Upon completion of the reaction, the catalyst was removed by filtration through a diatomaceous earth pad and the filtrate was concentrated under reduced pressure to afford tert-butyl [2-(2-aminoethoxy)ethyl]carbamate (58, 3.0 g). The resulting product can be used directly in the subsequent reaction without further purification. 1H NMR (400 MHz, MeOD) δ 3.48-3.45 (m, 4H), 3.23-3.20 (t, 2H), 2.77-2.75 (m, 2H), 1.43 (s, 9H).

2752-17-2
170 suppliers
$65.00/1 G

24424-99-5
867 suppliers
$13.50/25G
![Carbamic acid, [2-(2-aminoethoxy)ethyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/127828-22-2.gif)
127828-22-2
151 suppliers
$25.00/250mg
Yield:127828-22-2 71.5%
Reaction Conditions:
in tetrahydrofuran at 0 - 20;
Steps:
4.2. N-(tert-Butyloxycarbonyl)-2,2'-oxybis(ethylamine) (1)
This compound was synthesized according to the procedure described previously with slight modifications.25 To a cooled (0 °C) solution of 2,2'-oxybis(ethylamine) (10.0 mL, 94.1 mmol) in methanol (1000 mL) was added dropwise a solution of di-tert-butyl dicarbonate ((Boc)2O; 10.3 g, 47.0 mmol) in THF (300 mL), and the mixture was stirred for 1 h at the same temperature and additional 24 h at room temperature. After removing the solvent in vacuo, the residue was dissolved in 1 N NaOH (200 mL), and extracted with chloroform (300 mL × 3). The organic phases were combined, dried over anhydrous MgSO4. The solvent was removed in vacuo to provide 1 as a colorless oil (6.88 g, 71.5%). 1H NMR (CDCl3): δ 1.39 (9H, s, Boc), 2.79-2.82 (2H, t, CH2), 3.24-3.28 (2H, dd, CH2), 3.41-3.43 (2H, t, CH2), 3.44-3.47 (2H, t, CH2), 5.00 (1H, d, NH). ESI-MS (M+H)+: m/z 205, found: 205
References:
Suzuki, Hiroyuki;Kanai, Ayaka;Uehara, Tomoya;Guerra Gomez, Francisco L.;Hanaoka, Hirofumi;Arano, Yasushi [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 2,p. 978 - 984] Location in patent:experimental part
![Carbamic acid, N-[2-(2-azidoethoxy)ethyl]-, 1,1-dimethylethyl ester](/CAS/20200331/GIF/176220-30-7.gif)
176220-30-7
22 suppliers
inquiry
![Carbamic acid, [2-(2-aminoethoxy)ethyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/127828-22-2.gif)
127828-22-2
151 suppliers
$25.00/250mg

24424-99-5
867 suppliers
$13.50/25G
60792-79-2
115 suppliers
$36.00/250mg
![Carbamic acid, [2-(2-aminoethoxy)ethyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/127828-22-2.gif)
127828-22-2
151 suppliers
$25.00/250mg

24424-99-5
867 suppliers
$13.50/25G
![Carbamic acid, [2-(2-aminoethoxy)ethyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/127828-22-2.gif)
127828-22-2
151 suppliers
$25.00/250mg

302331-20-0
30 suppliers
$480.00/1 G
![Carbamic acid, [2-(2-aminoethoxy)ethyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/127828-22-2.gif)
127828-22-2
151 suppliers
$25.00/250mg