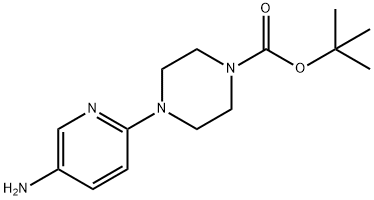
4-(5-AMINOPYRIDIN-2-YL)PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:4-(5-AMINOPYRIDIN-2-YL)PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:119285-07-3
- Molecular formula:C14H22N4O2
- Molecular Weight:278.35
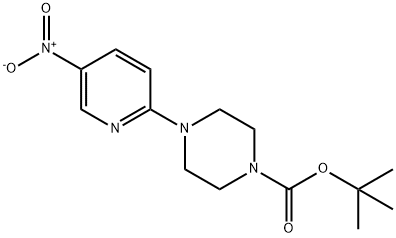
193902-78-2

119285-07-3
Example 197b Synthesis of tert-butyl 4-(5-aminopyridin-2-yl)piperazine-1-carboxylate 197b: To a 250-mL round-bottomed flask under nitrogen protection was added 1-tert-butylcarbonyl-4-(5-nitro-2-piperidinyl)piperazine 197a (4.0 g, 13.0 mmol), 10% palladium-carbon (wetted, 500 mg) and methanol (130 mL). The reaction system was evacuated and filled with hydrogen and the reaction was stirred at room temperature for 15 hours. Upon completion of the reaction, the hydrogen was removed by evacuation and filled with nitrogen. The reaction mixture was filtered through a diatomaceous earth pad to remove the catalyst and the filtrate was concentrated under reduced pressure to afford the target compound 197b (3.3 g, 91% yield). Mass spectral data (MS-ESI): [M + H]+ 279.

193902-78-2
79 suppliers
$55.00/250 mg

119285-07-3
106 suppliers
$22.00/100mg
Yield: 100%
Reaction Conditions:
with hydrogen;nickel in tetrahydrofuran under 2585.81 Torr; for 5 h;
Steps:
5; 8 4-(5-Amino-pyridin-2-yl)-piperazine-1-carboxylic acid tert-butyl ester
2-Bromo-5-nitro-pyridine (11.39 g, 56.1 mmol), tetrabutylammonium iodide (TBAI) (1.04 g, 0.05 mmol), potassium carbonate (8.53 g, 61.7 mmol) and piperazine-1-carboxylic acid tert-butyl ester (11.5 g, 61.7 mmol) were mixed together in DMSO (100 mL) and gently warmed to 50 C. for 3 hours and cooled to room temperature overnight. The reaction was diluted with EtOAc (200 mL), the salts were filtered and then the EtOAc was evaporated to leave the DMSO solution. This was diluted with water and a precipitate formed. This precipitate was filtered, washed with water, and then dried in an oven vacuum to give 4-(5-nitro-pyridin-2-yl)-piperazine-1-carboxylic acid tert-butyl ester (16.1 g, 93%) as a light orange solid. 1H NMR (400 MHz, CDCl3) ppm 1.47 (s, 9H), 3.55 (m, 4H), 3.75 (m, 4H), 6.55 (d, J=9.3 Hz, 1H), 8.21 (dd, J=9.5, 2.7 Hz, 1H), 9.03 (d, J=2.7 Hz, 1H). 4-(5-Nitro-pyridin-2-yl)-piperazine-1-carboxylic acid tert-butyl ester (16.0 g, 51.9 mmol) was dissolved in THF (400 mL), RaNi (4 g) added and placed under a H2 atmosphere at 50 psi for 5 h. The catalyst was removed by filtration through celite and the solvent evaporated in vacuo to give 4-(5-amino-pyridin-2-yl)-piperazine-1-carboxylic acid tert-butyl ester (14.5 g, 100%). 1H NMR (400 MHz, CDCl3) ppm 1.46 (s, 9H), 3.31 (m, 6H), 3.53 (m, 4H), 6.56 (d, J=8.8 Hz, 1H), 6.98 (dd, J=8.8, 2.9 Hz, 1H), 7.78 (dd, J=2.9, 0.7 Hz, 1H). m/z 279.1 (M+1).
References:
WARNER-LAMBERT COMPANY, LLC US2005/182078, 2005, A1 Location in patent:Page/Page column 9; 12

4548-45-2
555 suppliers
$6.00/5g

119285-07-3
106 suppliers
$22.00/100mg
57260-71-6
741 suppliers
$5.00/5g

119285-07-3
106 suppliers
$22.00/100mg

4487-59-6
448 suppliers
$5.00/1g

119285-07-3
106 suppliers
$22.00/100mg

4548-45-2
555 suppliers
$6.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

57260-71-6
741 suppliers
$5.00/5g

119285-07-3
106 suppliers
$22.00/100mg