
3-Quinolinecarboxylic acid, 1-cyclopropyl-5,6,7,8-tetrafluoro-1,4-dihydro-4-oxo-, ethyl ester synthesis
- Product Name:3-Quinolinecarboxylic acid, 1-cyclopropyl-5,6,7,8-tetrafluoro-1,4-dihydro-4-oxo-, ethyl ester
- CAS Number:107564-02-3
- Molecular formula:C15H11F4NO3
- Molecular Weight:329.25
![Benzenepropanoic acid, α-[(cyclopropylamino)methylene]-2,3,4,5,6-pentafluoro-β-oxo-, ethyl ester](/CAS/20211123/GIF/107564-01-2.gif)
107564-01-2
2 suppliers
inquiry

107564-02-3
7 suppliers
inquiry
Yield:107564-02-3 96.9%
Reaction Conditions:
with potassium phosphate in acetonitrile at 75 - 80; for 1.5 h;
Steps:
1 Example 1: Preparation of ethyl 1-cyclopropyl-5, 6,7, 8-tetrafluoro-1, 4-dihydro-4-oxoquinoline-3-carboxylate
3.0 g of ethyl 3-cyclopropylamino-2-pentafluorobenzoyl acrylate was dissolved in 15 ml of acetonitrile under heating to 75-80 °C. 3.28 g (1.8 eq. ) of K3PO4 was added in portions to the reaction mixture, which was then stirred at the same temperature for 1.5 hours. The reaction mixture was filtered under a reduced pressure and washed with 30 ml of DICHLOROMETHANE. The filtrate was concentrated under a reduced pressure. The resulting residue was dissolved in 30 mi of DICHLOROMETHANE and then washed with water. The organic layer was concentrated under a reduced pressure to give 2.74g of ethyl 1-CYCLOPROPYL-5, 6,7, 8-tetrafluoro-1, 4-dihydro-4-oxoquinoline-3-carboxylate (Yield : 96.9 %). 1H NMR (CDC13, PPM) : 1.17 (4H, m, CH2CH2), 1.39 (3H, t, J=8, CH2CH3), 3.88 (1 H, m, NCH), 4.37 (2H, q, J=8, CH2CH3), 8.48 (1 H, S, C2-H)
References:
WO2004/56781,2004,A1 Location in patent:Page 5
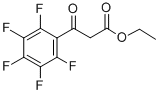
3516-87-8
92 suppliers
$55.00/100mg

122-51-0
513 suppliers
$10.00/5ml
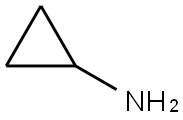
765-30-0
498 suppliers
$10.00/25g

107564-02-3
7 suppliers
inquiry

114226-95-8
2 suppliers
inquiry

107564-02-3
7 suppliers
inquiry

602-94-8
321 suppliers
$7.00/10g

107564-02-3
7 suppliers
inquiry

2251-50-5
237 suppliers
$7.00/1g

107564-02-3
7 suppliers
inquiry