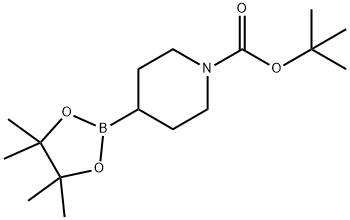
tert-butyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)piperidine-1-carboxylate
- CAS Number:1048970-17-7
- Molecular formula:C16H30BNO4
- Molecular Weight:311.22

375853-82-0

1048970-17-7
General procedure for the synthesis of 1-N-tert-butoxycarbonylpiperidine-4-boronic acid pinacol ester from 1,2,3,6-tetrahydro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine: method 4: general conditions for the reduction of tetrahydropyridines to piperidines in the presence of boronic acid ester: the boronic acid ester was dissolved in a mixed solvent of ethyl acetate and methanol (1:1 v/v) , with a final borate concentration of 0.4 M) in a mixture of ethyl acetate and methanol, followed by the addition of palladium hydroxide (0.35 equiv). The reaction mixture was stirred under hydrogen atmosphere for 14 hours. Upon completion of the reaction, the reaction mixture was filtered and concentrated in vacuum to give the target piperidine product in quantitative yield. Example 8: Piperidine 8 was prepared from compound 1 in a two-step reaction using Method 4, followed by deprotection of pinacol esters using Method 2. [M-H]? = 228.2 m/z. activity: b

76-09-5
393 suppliers
$8.19/250mg
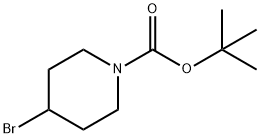
180695-79-8
191 suppliers
$14.00/1g

274-07-7
163 suppliers
$78.09/5g

1048970-17-7
153 suppliers
$11.00/250mg
Yield:1048970-17-7 36%
Reaction Conditions:
Stage #1:benzo[1,3,2]dioxaborole with bis(cyclopentadienyl)titanium dichloride;potassium carbonate in tert-butyl methyl ether for 0.5 h;
Stage #2:tert-butyl 4-bromo-1-piperidinecarboxylate in tert-butyl methyl ether at 80; under 760.051 Torr; for 24 h;Inert atmosphere;
Stage #3:2,3-dimethyl-2,3-butane diol with triethylamine in tert-butyl methyl ether at 20; for 1 h;Inert atmosphere;
Steps:
80 Example 80
bis(cyclopentadiene)titanium dichloride (denoted as Cp2TiCl2, 0.01mmol, 2.5mg), potassium carbonate (denoted as K2CO3, 0.2mmol, 26.7mg), methyl tert-butyl ether (1mL) Add catecholborane (denoted as HBcat, 0.6mmol, 63μL) into a 38mL pressure tube in sequence, stir for 30min and then add 1-Boc-4-bromopiperidine (denoted as 1ao, 0.2mmol,53mg) in nitrogen Stir at 80°C for 24h under a (1atm) atmosphere, add triethylamine (0.6mmol, 84μL) and pinacol (0.6mmol, 70.8mg) after the reaction, and stir at room temperature for 1h. Then use petroleum ether/ethyl acetate (20:1, v/v) as the eluent to carry out column chromatography purification to obtain the structure compound represented by formula 2ao (colorless and transparent liquid, 1-tert-butyl carboxylate-2-(4) ,4,5,5-Tetramethyl-1,3,2-dioxaborocyclyl)piperidine). The isolated yield is 36%.
References:
CN112645971, 2021, A Location in patent:Paragraph 0229-0231

73183-34-3
583 suppliers
$6.00/5g

180695-79-8
191 suppliers
$14.00/1g

1048970-17-7
153 suppliers
$11.00/250mg
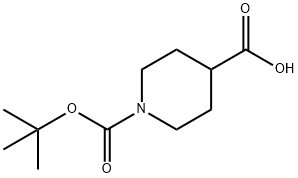
84358-13-4
378 suppliers
$5.00/5g

1048970-17-7
153 suppliers
$11.00/250mg

24424-99-5
859 suppliers
$13.50/25G
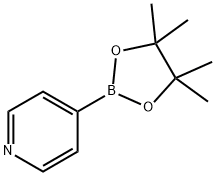
181219-01-2
244 suppliers
$13.00/1g

1048970-17-7
153 suppliers
$11.00/250mg

76-09-5
393 suppliers
$8.19/250mg

120347-72-0
17 suppliers
inquiry

24424-99-5
859 suppliers
$13.50/25G

1048970-17-7
153 suppliers
$11.00/250mg