
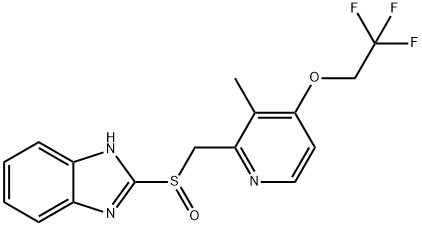
2-[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methylthio-1H-benzimidazole synthesis
- Product Name:2-[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methylthio-1H-benzimidazole
- CAS Number:103577-40-8
- Molecular formula:C16H14F3N3OS
- Molecular Weight:353.36

128430-66-0
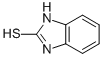
134469-07-1
![2-[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methylthio-1H-benzimidazole](/CAS/GIF/103577-40-8.gif)
103577-40-8
2-Chloromethyl-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine (91 g, 0.38 mol) and 1H-benzo[d]imidazole-2-thiol (57 g, 0.38 mol) were used as raw materials, suspended in methanol (1 L), and benzyltributylammonium bromide (2.15 g) was added as a phase transfer catalyst. A 30% NaOH solution (65 mL) was added slowly and dropwise over a period of 1 h, followed by stirring the reaction for 2 h at room temperature. Upon completion of the reaction, water (300 mL) was added to the reaction mixture and stirring was continued for 30 min. The mixture was cooled to 10 °C and the pH was adjusted to 9 with 35% HCl, at which point the target product 2-(((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methyl)thio)-1H-benzo[d]imidazole precipitated as a precipitate. The precipitate was collected by filtration and washed sequentially with 50% methanol and water. Finally, the product was dried under vacuum at 50 °C to give 129 g in 96% yield.

128430-66-0
106 suppliers
$113.00/250 mg

134469-07-1
28 suppliers
inquiry
![2-[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methylthio-1H-benzimidazole](/CAS/GIF/103577-40-8.gif)
103577-40-8
392 suppliers
$7.00/250mg
Yield:103577-40-8 96%
Reaction Conditions:
with benzyl tri-n-butylammonium bromide;sodium hydroxide in methanol at 20; for 3 h;Reagent/catalyst;
Steps:
1.2 (2) Preparation of 2 - [[2- (3-methyl-4- (2,2,2-trifluoroethoxy) pyridyl) methyl] -thio] -1H-benzimidazole
2-chloromethyl-3-methyl-4- (2,2,2-trifluoroethoxy) pyridine (91 g, 0.38 mol)2-mercaptobenzimidazole (57 g, 0.38 mol),Suspended in methanol (1 L)Benzyltributylammonium bromide (2.15 g, 0.006 mol)A 30% NaOH solution (65 mL) was added dropwise over 1 hour,Room temperature reaction 2h.To the mixture was added 300 mL of water,Stir for 30 minutes.10 ° C with 35% HCl adjusted to pH 9,Precipitation precipitation.filter,Followed by washing with 50% methanol and water.Dried at 50 ° C under vacuum,To give 2 - [[2- (3-methyl-4- (2,2,2-trifluoroethoxy) pyridin-yl) methyl] - thio] -1H- benzimidazole-129g,Yield 96%.
References:
Shijiazhuang Du Zhi Pharmaceutical Technology Co., Ltd.;Fang Yu;Du Yumin CN106543146, 2017, A Location in patent:Paragraph 0025; 0031; 0032; 0041; 0045; 0049; 0053-0069
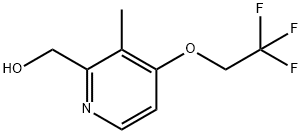
103577-45-3
823 suppliers
$7.00/1g
![2-[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methylthio-1H-benzimidazole](/CAS/GIF/103577-40-8.gif)
103577-40-8
392 suppliers
$7.00/250mg

103577-61-3
58 suppliers
inquiry
![2-[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methylthio-1H-benzimidazole](/CAS/GIF/103577-40-8.gif)
103577-40-8
392 suppliers
$7.00/250mg
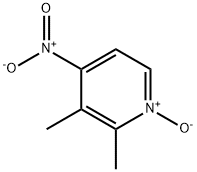
103577-66-8
245 suppliers
$7.00/1g
![2-[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methylthio-1H-benzimidazole](/CAS/GIF/103577-40-8.gif)
103577-40-8
392 suppliers
$7.00/250mg
37699-43-7
398 suppliers
$10.00/1g
![2-[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methylthio-1H-benzimidazole](/CAS/GIF/103577-40-8.gif)
103577-40-8
392 suppliers
$7.00/250mg