
1-TrifluoroMethyl-1,2-benziodoxol-3(1H)-one synthesis
- Product Name:1-TrifluoroMethyl-1,2-benziodoxol-3(1H)-one
- CAS Number:887144-94-7
- Molecular formula:C8H4F3IO2
- Molecular Weight:316.02
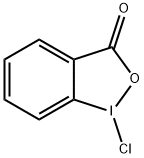
59457-26-0

81290-20-2

887144-94-7
General procedure for the synthesis of 1-(trifluoromethyl)-1,2-phenyliodonyl-3(1H)-ones from 1-chloro-1,2-phenyliodonyl-3-one and (trifluoromethyl)trimethylsilane: Potassium acetate (10.8 g, 0.110 mol, 2.00 eq.) was dried under vacuum at 150 °C for 30 min and subsequently cooled to room temperature. To this was added 1-chloro-1,2-phenyliodono-3-one (15.6 g, 0.0551 mol, 1.00 eq.) and acetonitrile (155 mL), and the reaction mixture was stirred at 75 °C for 2 hours. After completion of the reaction, the mixture was cooled to room temperature. (Trifluoromethyl)trimethylsilane (8.97 mL, 0.0607 mol, 1.10 eq.) was added all at once and the reaction mixture was stirred at room temperature for 15 hours. Subsequently, acetonitrile (100 mL) was added and the brown reaction mixture was heated to 75 °C. The reaction mixture was filtered through a diatomaceous earth pad, concentrated to a final volume of about 50 mL and cooled to -20 °C. The crystals were filtered out, washed with cold (-20 °C) acetonitrile (50 mL) and dried under vacuum. The mother liquor was reconcentrated to a final volume of about 15 mL and cooled to -20 °C. The crystals were filtered out and washed with cold (-20°C) acetonitrile (10 mL). Both crystallization products were dried under high vacuum to afford 1-(trifluoromethyl)-1,2-phenyliodonyl-3(1H)-one as a white crystalline solid (14.6 g, 0.0463 mol, 84% yield). The NMR data were as follows: 1H NMR (500 MHz, CDCl3, 25 °C, δ): 8.48 (dd, J = 7.2, 1.7 Hz, 1H), 7.85-7.76 (m, 3H). 13C NMR (175 MHz, CDCl3, 25 °C, δ): 165.9, 135.9, 133.9, 132.1, 132.0 , 127.3 (q, J = 2.5 Hz), 114.9, 107.3 (q, J = 378.8 Hz).19F NMR (376 MHz, CDCl3, 25 °C, δ): -34.2 (s). These spectral data are in agreement with literature reports.

59457-26-0
39 suppliers
$80.00/250mg

81290-20-2
420 suppliers
$13.00/1g

887144-94-7
184 suppliers
$19.00/1g
Yield:887144-94-7 84%
Reaction Conditions:
Stage #1:1-chloro-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one with potassium acetate in acetonitrile at 75; for 2 h;
Stage #2:(trifluoromethyl)trimethylsilane in acetonitrile at 20; for 15 h;
Steps:
1-(trifluoromethyl)-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one
Potassium acetate (10.8g, 0.110 mol, 2.00 equiv) was dried under vacuum at 150°C for 30 min and then cooled to rt. l-chloro-lX3- benzo [d] [1, 2] iodaoxol-3 (1H) -one (15.6 g, 0.0551 mol, 1.00 equiv) and MeCN (155 mL) were added and the reaction mixture was stirred at 75°C for 2h. The reaction mixture was cooled to rt. T SCF3 (8.97 mL, 0.0607 mol, 1.10 equiv) was added in one portion and the reaction mixture was stirred at rt for 15 h.MeCN (100 mL) was then added and the brown reaction mixture was heated to 75°Cand the hot reaction mixture was filtered through a pad of Celite, concentrated to about 50 mL end volume, and cooled to -20°C. The crystals were filtered off, washed with cold (-20 °C) MeCN (50 mL) and dried under vacuum. The mother liquor was again concentrated to approximately 15 mL end volume and cooled to -20°C. The crystals were filtered off and washed with a cold (-20°C) MeCN (10 mL) . Both crystalline fractions were dried under a high vacuum to afford the title compound as a white crystalline solid (14.6 g, 0.0463 mol, 84% yield). NMR Spectroscopy: lH NMR (500 MHz, CDCI3, 25°C, δ):8.48 (dd, J = 7.2, 1.7 Hz, 1H) , 7.85- 7.76 (m, 3H) . 13C NMR (175 MHz, CDCI3, 25°C, δ) : 165.9, 135.9, 133.9, 132.1, 132.0, 127.3 (q, J= 2.5 Hz), 114.9, 107.3 (q, J= 378.8Hz) .19F NMR (376 MHz, CDCI3, 25°C, δ) : -34.2(s). These spectroscopic data correspond to previously reported data.
References:
THE RESEARCH FOUNDATION FOR THE STATE UNIVERSITY OF NEW YORK;NGAI, Ming-Yu;HOJCZYK, Katarzyna, N. WO2016/57931, 2016, A1 Location in patent:Page/Page column 55

1829-26-1
36 suppliers
inquiry

81290-20-2
420 suppliers
$13.00/1g

887144-94-7
184 suppliers
$19.00/1g
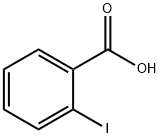
88-67-5
564 suppliers
$5.00/5g

81290-20-2
420 suppliers
$13.00/1g

887144-94-7
184 suppliers
$19.00/1g

81290-20-2
420 suppliers
$13.00/1g

1829-25-0
17 suppliers
inquiry

887144-94-7
184 suppliers
$19.00/1g
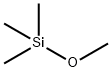
1825-61-2
251 suppliers
$15.00/25g

88-67-5
564 suppliers
$5.00/5g

887144-94-7
184 suppliers
$19.00/1g