
1-N-Boc-3-Azetidinecarboxylic acid synthesis
- Product Name:1-N-Boc-3-Azetidinecarboxylic acid
- CAS Number:142253-55-2
- Molecular formula:C9H15NO4
- Molecular Weight:201.22

24424-99-5

36476-78-5

142253-55-2
General procedure for the synthesis of 1-Boc-azetidine-3-carboxylic acid from di-tert-butyl dicarbonate and 3-acridinecarboxylic acid: Example 4 Synthesis of 6-(3-{[(benzylsulfonyl)amino]carbonyl}azetidin-1-yl)-5-cyano-2-(difluoromethyl)nicotinate (a) Preparation of ethyl 1-(tert-butoxycarbonyl)azetidine-3-carboxylate Di-tert-butyl dicarbonate (25.535 g, 117 mmol) dissolved in methanol (70 mL) was added dropwise to a stirred solution of azetidine-3-carboxylic acid (10.11 g, 100 mmol) and triethylamine (27.8 mL, 200 mmol) in methanol (105 mL) over a period of 20 min. The reaction was carried out at room temperature (mild exotherm was observed) with continuous stirring for 18 hours. Upon completion of the reaction, the mixture was evaporated to dryness, tetrahydrofuran (120 mL) was added and evaporated again to afford the crude 1-(tert-butoxycarbonyl)azetidine-3-carboxylic acid, which could be used in the next step without further purification. Yield: 25.89 g (128%). 1H NMR (400 MHz, CDCl3) δ 1.43 (9H, s), 3.21-3.34 (1H, m), 4.00-4.13 (4H, m).

24424-99-5
866 suppliers
$13.50/25G

36476-78-5
435 suppliers
$6.00/1g

142253-55-2
330 suppliers
$6.00/1g
Yield:142253-55-2 100%
Reaction Conditions:
with triethylamine in methanol at 20; for 18 h;
Steps:
4.a
Example 4Ethyl 6-(3-{[(benzylsuIfonyl)amino]carbonyl}azetidin-l-yl)-5-cyano-2- 30 (difluoromethyl)nicotinate(a) l-(tert-Butoxycarbonyl)azetidine-3-carboxylic acid
References:
WO2008/4946,2008,A1 Location in patent:Page/Page column 90-91

142253-54-1
180 suppliers
$9.00/250mg

142253-55-2
330 suppliers
$6.00/1g

24424-99-5
866 suppliers
$13.50/25G

102624-96-4
69 suppliers
$45.00/10mg

142253-55-2
330 suppliers
$6.00/1g

24424-99-5
866 suppliers
$13.50/25G

36476-78-5
435 suppliers
$6.00/1g

142253-55-2
330 suppliers
$6.00/1g
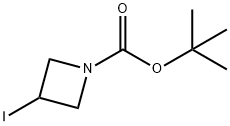
254454-54-1
265 suppliers
$15.00/1g

142253-55-2
330 suppliers
$6.00/1g