
(1-Methyl-1H-pyrazol-5-yl)-boronic acid synthesis
- Product Name:(1-Methyl-1H-pyrazol-5-yl)-boronic acid
- CAS Number:720702-41-0
- Molecular formula:C4H7BN2O2
- Molecular Weight:125.92
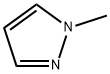
930-36-9

720702-41-0
General procedure for the synthesis of 1-methyl-1H-pyrazole-5-boronic acid from 1-methylpyrazole: 1-methylpyrazole (25 mL, 0.3 mol) was dissolved in 500 mL of tetrahydrofuran (THF). The solution was then cooled to -78°C in a dry ice/isopropanol bath. After the solution temperature stabilized at -78 °C, n-butyllithium (n-BuLi, 140 mL, 0.40 mol) was slowly added dropwise through the cannula. After the dropwise addition, the reaction mixture was stirred continuously at -78 °C for 1.5 hours. Next, triisopropyl borate (280 mL, 1.2 mol) was added to the reaction system via cannula and the reaction mixture was allowed to slowly warm from -78 °C to 0 °C while stirring overnight. Upon completion of the reaction, the pH of the mixture was adjusted to 6 with 1 N hydrochloric acid (HCl).Subsequently, THF was evaporated under reduced pressure and the remaining aqueous phase was extracted with ethyl acetate (EtOAc, 2 × 100 mL). Finally, the solid was collected by filtration to afford 108 g (100% yield) of 1-methyl-1H-pyrazole-5-boronic acid as a yellow solid.

930-36-9
335 suppliers
$5.00/1g

720702-41-0
176 suppliers
$12.00/250mg
Yield:720702-41-0 100%
Reaction Conditions:
Stage #1:1-methyl-1H-pyrazole with n-butyllithium in tetrahydrofuran at -78; for 1.5 h;
Stage #2: with Triisopropyl borate in tetrahydrofuran at -78 - 0;
Stage #3: with hydrogenchloride;water in tetrahydrofuran; pH=6
Steps:
1.1.A
2-Methyl-2H-pyrazole-3-boronic acid: N-methyl pyrazole (25 mL, 0.3 mol) was dissolved in 500 mL of THF. The solution was then cooled to-78°C in a dry ice/isopropanol bath. Once the solution reached -78°C, n-BuLi (140 mL, 0.40 mol) was added dropwise by canula. The reaction mixture was stirred at -78°C for 1.5 hours. Then, triisopropyl borate (280 mL, 1.2 mol) was added to the above mixture via canula. While stirring overnight, the reaction temperature was gradually increased from -78δC to 0δC. The pH of the mixture was adjusted to 6 with IN HCI. THF was removed under reduced pressure, and the aqueous residue was extracted with EtOAc (2 x 100mL). The solid was then filtered to yield 108 g (100%) of 2-methyl-2H-pyrazole-3-boronic acid as a yellow solid.
References:
ARENA PHARMACEUTICALS, INC. WO2005/12254, 2005, A1 Location in patent:Page/Page column 101

930-36-9
335 suppliers
$5.00/1g

5419-55-6
391 suppliers
$12.00/5g

720702-41-0
176 suppliers
$12.00/250mg

930-36-9
335 suppliers
$5.00/1g
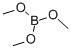
121-43-7
385 suppliers
$13.00/25mL

720702-41-0
176 suppliers
$12.00/250mg
