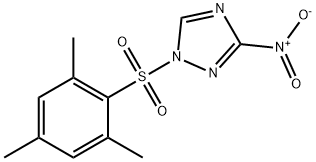
1-(Mesitylene-2-sulfonyl)-3-nitro-1,2,4-triazole synthesis
- Product Name:1-(Mesitylene-2-sulfonyl)-3-nitro-1,2,4-triazole
- CAS Number:74257-00-4
- Molecular formula:C11H12N4O4S
- Molecular Weight:296.3
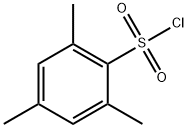
773-64-8
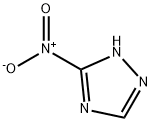
24807-55-4

74257-00-4
An oven-dried 1.0-liter round-bottomed flask equipped with a magnetic stirring bar was assembled with a Claisen adapter. To the reaction flask was added 3-nitro-1,2,4-triazole (19.5 g, 0.171 mol), anhydrous dioxane (200 mL), and triethylamine (17.3 g, 0.171 mol, 1.0 eq.), and the solution was cooled to 0°C. A 200 mL pressure-equalizing dosing funnel was assembled through the Claisen adapter, and a dioxane solution (150 mL) of homotrimethylbenzenesulfonyl chloride ( 37.4 g, 0.171 mol) of dioxane solution (150 mL) to the funnel. Slowly add the homotrimethylbenzenesulfonyl chloride solution dropwise to the reaction mixture. After the dropwise addition was completed, the reaction mixture was continued to be stirred for 0.5 hours, followed by warming to room temperature and continued stirring for 1 hour. After completion of the reaction, the precipitated triethylamine hydrochloride was removed by filtration and the filtrate was concentrated under reduced pressure to give a yellow solid. The solid was dissolved in dichloromethane (150 mL) and the organic layer was washed with water (150 mL). The organic layer was dried with anhydrous sodium sulfate, filtered and the solvent evaporated under reduced pressure to give a yellow solid. Recrystallization by boiling toluene (20-30 mL) followed by washing with ice-cold toluene and drying overnight at 1.9 mmHg gave 1-(2-trimethylbenzenesulfonyl)-3-nitro-1,2,4-triazole 24-25 g (46%-48% yield) as a pale yellow solid with a melting point of 131°C-133°C (literature values 130°C-132°C). Thin Layer Chromatography (TLC) Rf = 0.23 (unfolding agent: dichloromethane solution of 20% hexane, UV detection).1H NMR (300 MHz, CDCl3) δ: 8.84 (s, 1H), 7.07 (s, 2H), 2.69 (s, 6H), 2.34 (s, 3H).13C NMR (75 MHz, CDCl3) δ. 162.9, 147.5, 145.2, 142.5, 133.0, 127.9, 23.2, 21.3. IR (solid) νmax: 3126, 2983, 2947, 1597 cm-1. Calculated elemental values (C11H12N4O4S): C, 44.59; H, 4.08; N, 18.91. Measured values: C , 44.69; H, 3.88; N, 18.72. The purity of the product was determined to be 96% by reversed-phase high-performance liquid chromatography (RP-HPLC), with a retention time of tR = 8.41 min (detection wavelength 254 nm).
Yield:74257-00-4 48%
Reaction Conditions:
with triethylamine in 1,4-dioxane for 1.5 h;Cooling with ice;
Steps:
1-(Mesitylenesulfonyl)-3-nitro-1,2,4-triazole (3)
An oven dried 1.0 l round-bottomedflask containing a magnetic stir bar was equipped with a Claisen adapter. 3-Nitro-1,2,4-triazole (19.5 g, 0.171 mol), dry dioxane (200 ml) and triethylamine (1.0 equiv., 17.3 g,0.171 mol) was transferred to the reaction flask and the solution was cooled in an ice-bathwith magnetic stirring.47 The Claisen adapter was fitted with a 200 ml pressure-equalizingaddition funnel, containing a dioxane solution (150 ml) of mesitylenesulfonyl chloride(37.4 g, 0.171 mol). The solution of mesitylenesulfonyl chloride was added dropwise overa period of approx. 0.5 h and the final suspension was stirred for an additional 1 h andthen warmed to rt. After removal of the precipitated Et3N·HCl by filtration, the filtratewas concentrated in vacuo to give a yellow solid, which was dissolved in dichloromethane(150 ml) and the solution was washed with water (150 ml).48 The organic layer was driedover anhydrous Na2SO4 and evaporated in vacuo to give a yellow solid. Recrystallizationfrom boiling toluene (20-30 ml) followed by washing with ice-cold toluene and dryingovernight at 1.9 mmHg, provided 24-25 g (46%-48%) of the title compound as light yellow solid,49 mp. 131C-133C (lit.37 130C-132C). Rf = 0.23 (20% hexane in CH2Cl2, UV);1H NMR (300 MHz, CDCl3): δ 8.84 (s, 1H), 7.07 (s, 2H), 2.69 (s, 6H), 2.34 (s, 3H);13C NMR (75 MHz, CDCl3): δ 162.9, 147.5, 145.2, 142.5, 133.0, 127.9, 23.2, 21.3; IR(solid): 3126, 2983, 2947, 1597.Anal. Calcd. for C11H12N4O4S: C, 44.59; H, 4.08; N, 18.91. Found: C, 44.69; H,3.88; N, 18.72. The purity of the product (96%) established by RP-HPLC, tR = 8.41 min(254 nm).
References:
Petersen, Rico;Jensen, Jakob F.;Nielsen, Thomas E. [Organic Preparations and Procedures International,2014,vol. 46,# 3,p. 267 - 271]