1-(CHLOROMETHYL)-3,5-BIS(METHYLSULFONYL)BENZENE synthesis
- Product Name:1-(CHLOROMETHYL)-3,5-BIS(METHYLSULFONYL)BENZENE
- CAS Number:39552-81-3
- Molecular formula:C9H11NO2
- Molecular Weight:165.19

67-56-1
1197-55-3

39552-81-3
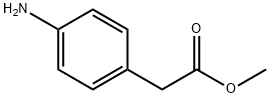
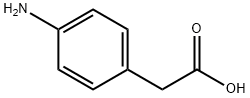
Synthesis of Intermediate 8a: Methyl 2-(4-aminophenyl)acetate 4-Aminophenylacetic acid (10.0 g, 66.6 mmol) was dissolved in methanol (350 mL), stirred and concentrated. Concentrated sulfuric acid (3.88 mL, 72.77 mmol) was added slowly and dropwise. The reaction mixture was stirred at 50 °C overnight and subsequently cooled to room temperature and concentrated under vacuum. The concentrated mixture was diluted with water, neutralized with sodium carbonate (Na2CO3) until the solid precipitated, and then extracted with dichloromethane (DCM). The organic layer was dried with anhydrous sodium sulfate (Na2SO4), filtered and concentrated to give methyl 2-(4-aminophenyl)acetate (9.75 g, 59.02 mmol, 89% yield). Method 2: RT: 0.60 min, m/z 165.9 [M + H]+.

1197-55-3
423 suppliers
$13.00/25g

39552-81-3
87 suppliers
$9.00/1g
Yield:39552-81-3 74%
Reaction Conditions:
with HCl conc.;sodium hydrogencarbonate in methanol;hexane;ethyl acetate
Steps:
Methyl-4-aminophenylacetate (3)
Methyl-4-aminophenylacetate (3) To a solution of 4-aminophenylacetic acid (10 g, 66.2 mmol) in methanol (150 mL) at room temperature was added HCl conc. (37% 25 mL). The mixture became yellow and was stirred overnight. The reaction mixture was then quenched with a saturated aqueous solution of NaHCO3. The methanol was evaporated under reduced pressure and the aqueous layer was extracted several times with AcOEt. The combined organic extracts were dried(MgSO4) and evaporated. The crude residue was purified by flash chromatography using hexane/AcOEt (4:1) as solvent mixture yielding 3 as a yellow oil (9.44 g, 74%). 1H NMR: (300 MHz, CDCl3): 7.05 (d, J=10 Hz, 2H), 6.65 (d, J=10 Hz, 2H), 3.65 (s, 3H), 3.63 (broad s, 2H), 3.51 (s, 2H).
References:
MethylGene, Inc. US6541661, 2003, B1
2945-08-6
68 suppliers
inquiry

39552-81-3
87 suppliers
$9.00/1g

67-56-1
782 suppliers
$7.29/5ml-f

1197-55-3
423 suppliers
$13.00/25g

39552-81-3
87 suppliers
$9.00/1g

1197-55-3
423 suppliers
$13.00/25g

18107-18-1
210 suppliers
$33.00/5g

39552-81-3
87 suppliers
$9.00/1g

104-03-0
448 suppliers
$5.00/10g

39552-81-3
87 suppliers
$9.00/1g