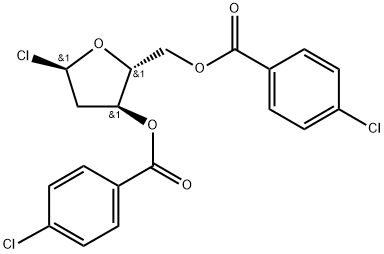
1-Chloro-3,5-di-(4-chlorobenzoyl)-2-deoxy-D-ribose synthesis
- Product Name:1-Chloro-3,5-di-(4-chlorobenzoyl)-2-deoxy-D-ribose
- CAS Number:21740-23-8
- Molecular formula:C19H15Cl3O5
- Molecular Weight:429.67

80339-59-9

21740-23-8
Example 3: Preparation of 1-[3,5-di-O-(p-chlorobenzoyl)]-2-deoxy-α-D-ribofuranosyl chloride To a 250 mL four-necked round-bottomed flask under nitrogen protection was added 1-O-methyl-3,5-di-O-(p-chlorobenzoyl)-2-deoxy-α/β-D-ribofuranose (15.94 g), glacial acetic acid (60 mL), anhydrous chloroform (30 mL), anhydrous dioxane (30 mL) and acetyl chloride (0.87 g). Under stirring conditions, gaseous HCl (11.5 g) was slowly passed while the reaction temperature was controlled between 13 and 15 °C. During this process, 1-[3,5-di-O-(p-chlorobenzoyl)]-2-deoxy-α-D-ribofuranosyl chloride was gradually crystallized and precipitated.After the completion of HCl passage, the reaction mixture was continued to be stirred at 12 °C for 20 min. Subsequently, the product was collected by filtration and washed with anhydrous hexane (20 mL). After drying, 12.4 g of the target compound 1-[3,5-di-O-(p-chlorobenzoyl)]-2-deoxy-α-D-ribofuranosyl chloride was obtained as a white powder in 77% yield.

80339-59-9
1 suppliers
inquiry

21740-23-8
228 suppliers
$21.00/1g
Yield:21740-23-8 77%
Reaction Conditions:
with hydrogenchloride;acetic acid;acetyl chloride in 1,4-dioxane;chloroform at 13 - 15;Inert atmosphere;
Steps:
3
Example 3 Preparation of 1-[3,5-di-O-(p-chlorobenzoyl)]-2-deoxy-alpha-D-ribofuranosyl-chloride.1-O-methyl-3,5-di-O-p-chlorobenzoyl-2-deoxy-alpha/beta-D-ribose (15.94 g), glacial acetic acid (60 mL), anhydrous chloroform (30 mL), anhydrous dioxane (30 mL) and acetyl chloride (0.87 g) were added into a 4-neck, 250 mL round-bottom flask. Under stirring and nitrogen, gaseous HCl was fed (11.5 g) subsurface while the temperature was maintained between 13 to 15° C. 1-[3,5-di-O-(p-chlorobenzoyl)]-2-deoxy-alpha-D-ribofuranosyl-chloride slowly crystallized out during HCl feeding. The slurry was stirred at 12° C. for 20 minutes after HCl feeding was complete. The product was collected and rinsed with anhydrous hexanes (20 mL). After drying, 12.4 g of 1-[3,5-di-O-(p-chlorobenzoyl)]-2-deoxy-alpha-D-ribofuranosyl-chloride was isolated as white powder (77% yield).
References:
US2010/249394,2010,A1 Location in patent:Page/Page column 4-5