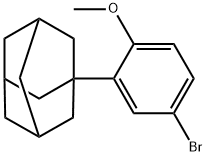
1-(5-Bromo-2-methoxy-phenyl)adamantane synthesis
- Product Name:1-(5-Bromo-2-methoxy-phenyl)adamantane
- CAS Number:104224-63-7
- Molecular formula:C17H21BrO
- Molecular Weight:321.25
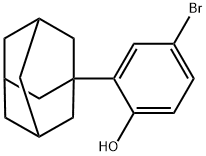
104224-68-2

74-88-4

104224-63-7
To a stirred mixture of anhydrous N,N-dimethylformamide (5 ml) containing 2-(1-adamantyl)-4-bromophenol (0.5 g; 1.62 mmol) and anhydrous potassium carbonate (0.335 g; 2.42 mmol), iodomethane (1 ml) was added. The reaction mixture was stirred at room temperature for 2 hours. After completion of the reaction, the mixture was diluted to 100 ml with distilled water and extracted with ethyl acetate (100 ml). The organic layers were combined, dried with anhydrous magnesium sulfate and filtered to remove the desiccant. The filtrate was filtered through silica gel column to remove impurities. Finally, the filtrate was concentrated to dryness under reduced pressure to give 1-(5-bromo-2-methoxyphenyl)adamantane (0.49 g, 94% yield) as a light yellow-green solid.1H-NMR (CDCl3) δ: 7.27-7.22 (m, 2H); 6.31 (d, 1H, J = 6.5 Hz); 3.78 (s, 3H); 2.04 (s, 10H). 1.74 (br, 5H).
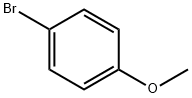
104-92-7
436 suppliers
$10.00/10g

768-95-6
453 suppliers
$10.00/5g

104224-63-7
311 suppliers
$16.00/5g
Yield:104224-63-7 75%
Reaction Conditions:
with sulfuric acid in dichloromethane at 20; for 20 h;
Steps:
1
1-(5-Bromo-2-methoxyphenyl)-adamantane [0145] [0146] Reagent grade concentrated H2S04 (11 mL) was added dropwise to a solution of 1- adamantol (30.25 g, 200 mmol) and 4-bromoanisole (37.21g, 200 mmol) in 130 mL of CH2Cl2. The light pink solution was stirred at ambient temperature for 20 hours. The solvent was decanted, water (100 mL) and hexane (100 mL) were added and the solid was filtered and washed with hexane and dried to give 31 g of the product as a white powder. The supernatant was diluted with hexane, washed with water and brine, dried over MgS04 and filtered thru a silica gel pad. The solvent was removed and the solid was recrystallized from hexane to yield 17 g of the product as a white powder. [0147] Yield: 65 g (75%) ; white solid; Rf= 0.9 in 25% EtOAc-hexane. 1H NMR (CDC13, 300 MHz) No. 1.78 (s, 6H), 2.08 (s, 9H), 3.81 (s, 3H), 6.72 (d, 1H), 7.24 (dd, 1H), 7.28 (m, 1H)
References:
AUSPEX PHARMACEUTICALS WO2005/108338, 2005, A1 Location in patent:Page/Page column 47

104224-68-2
142 suppliers
$50.00/2 g

74-88-4
356 suppliers
$15.00/10g

104224-63-7
311 suppliers
$16.00/5g

77-78-1
325 suppliers
$19.69/1ml

104224-68-2
142 suppliers
$50.00/2 g

104224-63-7
311 suppliers
$16.00/5g

768-95-6
453 suppliers
$10.00/5g

104224-63-7
311 suppliers
$16.00/5g
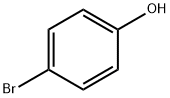
106-41-2
503 suppliers
$13.00/5g

104224-63-7
311 suppliers
$16.00/5g