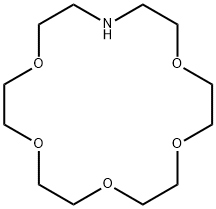
1,4,7,10,13-PENTAOXA-16-AZACYCLOOCTADECANE synthesis
- Product Name:1,4,7,10,13-PENTAOXA-16-AZACYCLOOCTADECANE
- CAS Number:33941-15-0
- Molecular formula:C12H25NO5
- Molecular Weight:263.33
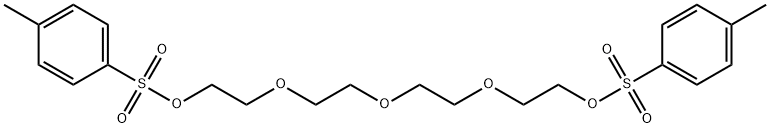
37860-51-8
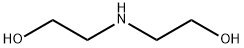
111-42-2

33941-15-0
To a 3L three-necked round-bottomed flask was sequentially added a magnetic stirrer, 1.50 L of tert-butanol (tBuOH) and potassium tert-butanol (KOtBu, 30.7 g, 274 mmol). The mixture was heated to 40 °C with continuous stirring for 30 min to ensure complete dissolution of KOtBu. Subsequently, tetraethylene glycol di-p-toluenesulfonate (46 g, 91.5 mmol, dissolved in 100 mL of dioxane) and diethanolamine (9.6 g, 91.3 mmol, dissolved in 100 mL of tBuOH), respectively, were slowly added dropwise to the reaction system over a period of 2 hours through two different dropping funnels. Note: The rate of titration has a significant effect on the reaction yield, and slow titration helps to increase the yield. After dropwise addition, the reaction mixture was continued to be stirred for 1 hour and subsequently cooled to room temperature. The reaction mixture was filtered through a Brinell funnel and the solvent was removed by rotary evaporator. To the residual brown viscous mass was added 100 mL of deionized water and extracted first with hexane (1 x 60 mL, discarding the hexane phase) and then with dichloromethane (CH2Cl2, 5 x 60 mL). The dichloromethane phases were combined, dried with anhydrous magnesium sulfate (MgSO4) and the solvent was again removed by rotary evaporator. The resulting dark brown residue was distilled under high vacuum through a ball-bulb distillation apparatus using a hot air gun for assisted heating, resulting in the colorless liquid-like product az-18-crown ether-6 (8.4 g, 35% yield). The nuclear magnetic resonance (NMR) spectrum of the product was consistent with the data reported in the literature. It was found that the tBuOH used in the reaction could be recovered by distillation and used in the subsequent synthesis of aza-crown ether even though it contained a small amount of dioxane (<5%).
![Bis[2-(2-hydroxyethoxy)ethyl] ether](/CAS/GIF/112-60-7.gif)
112-60-7
370 suppliers
$7.00/25g

33941-15-0
94 suppliers
$38.00/250mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1: 96 percent / aq. KOH / tetrahydrofuran / 10 h / 0 - 20 °C
2: 24 percent / sodium tert-butoxide / 2-methyl-propan-2-ol; dioxane / 1 h / 20 - 40 °C
References:
Fallouh;Bernier;Virieux;Cristau;Pirat [Phosphorus, Sulfur and Silicon and the Related Elements,2006,vol. 181,# 1,p. 219 - 225]
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

33941-15-0
94 suppliers
$38.00/250mg

37860-51-8
164 suppliers
$6.00/1g

111-42-2
865 suppliers
$10.00/20mg

33941-15-0
94 suppliers
$38.00/250mg

118798-05-3
1 suppliers
inquiry

33941-15-0
94 suppliers
$38.00/250mg

85971-97-7
0 suppliers
inquiry

33941-15-0
94 suppliers
$38.00/250mg