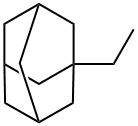
1,3-Dimethyladamantane synthesis
- Product Name:1,3-Dimethyladamantane
- CAS Number:702-79-4
- Molecular formula:C12H20
- Molecular Weight:164.29

941-37-7

702-79-4
GENERAL METHODS: A thiocarbonate solution was prepared by dissolving 5 g (107 mg, 0.200 mmol) of 1-bromo-3,5-dimethyladamantane in CPME (0.5 mL). Subsequently, a solution of n-Bu3SnH (75.7 mg, 0.260 mmol) and AIBN (6.6 mg, 0.040 mmol) in CPME (1.5 mL) was slowly added dropwise by syringe pump to the above thiocarbonate solution, and the reaction lasted for 30 min at 90 °C. After completion of the reaction, the mixture was continued to be stirred at the same temperature for 30 min, followed by concentration. The residue was purified by 10% w/w K2CO3-silica gel fast column chromatography using gradient elution (hexane → hexane/EtOAc = 100/1 → 50/1 → 20/1) to afford 6 g of cis-consistently cyclized product (53.6 mg, 0.140 mmol, 70% yield) and a mixture of cis- and trans-consistently diastereoisomers (22.0 mg. 0.0572 mmol, 29% yield, cis-trans ratio of 2.8:1). For spectral analysis, these isomers were separated by repeated column chromatography.

941-37-7
428 suppliers
$26.00/5g

702-79-4
469 suppliers
$5.00/1g
Yield:702-79-4 100%
Reaction Conditions:
with 2,2'-azobis(isobutyronitrile);tri-n-butyl-tin hydride at 90; for 1 h;Inert atmosphere;Barton-McCombie Deoxygenation;Solvent;
Steps:
4.6 General procedure for Barton-McCombie radical reduction of compound 5
General procedure: To a solution of thiocarbonate 5g (107 mg, 0.200 mmol) in CPME (0.5 mL) were added dropwise a solution of n-Bu3SnH (75.7 mg, 0.260 mmol) and AIBN (6.6 mg, 0.040 mmol) in CPME (1.5 mL) with a syringe pump over 30 min at 90 °C. The resultant mixture was stirred at 90 °C for 30 min and concentrated. The residue was purified by 10% w/w K2CO3-silica gel flash column chromatography (hexane→hexane/EtOAc=100/1→50/1→20/1) to give the cis-fused cyclized product 6g (53.6 mg, 0.140 mmol, 70%) and 2.8:1 mixture of cis and trans-fused diastereomers (22.0 mg, 0.0572 mmol, 29%). These isomers were separated by the repeated column chromatography for spectral analysis.
References:
Kobayashi, Shoji;Kuroda, Hiroyuki;Ohtsuka, Yuta;Kashihara, Takashi;Masuyama, Araki;Watanabe, Kiyoshi [Tetrahedron,2013,vol. 69,# 10,p. 2251 - 2259]

2146-36-3
93 suppliers
inquiry

702-79-4
469 suppliers
$5.00/1g
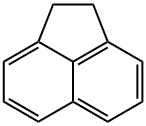
83-32-9
274 suppliers
$14.00/5g

702-79-4
469 suppliers
$5.00/1g
![Tricyclo[3.3.1.13,7]decane, 1-iodo-3,5-dimethyl-](/CAS/20210305/GIF/80405-15-8.gif)
80405-15-8
0 suppliers
inquiry

702-79-4
469 suppliers
$5.00/1g