
1,3-Dichloro-2-fluorobenzene synthesis
- Product Name:1,3-Dichloro-2-fluorobenzene
- CAS Number:2268-05-5
- Molecular formula:C6H3Cl2F
- Molecular Weight:164.99

2106-49-2

2268-05-5
The general procedure for the synthesis of 2,6-dichlorofluorobenzene from 3-chloro-2-fluoronitrobenzene is as follows: In a 250 ml four-necked flask, 240 g (1.368 mol) of 3-chloro-2-fluoronitrobenzene was added and the temperature was raised to 180°C. Dry chlorine gas was passed through the flask, and the temperature of the reaction was controlled to be between 180 and 190°C. The reaction was carried out by distillation. Distillation was carried out during the reaction for 8 to 10 hours. The fractions are collected and sequentially washed with water and alkali to a weak base. The organic phase was separated and subjected to vacuum distillation, resulting in 210 g of 2,6-dichlorofluorobenzene with 99.7% purity and 93% yield.

2106-49-2
187 suppliers
$5.00/5g

2268-05-5
237 suppliers
$5.00/5g
Yield:2268-05-5 93%
Reaction Conditions:
with chlorine at 180 - 190;Temperature;
Steps:
7 example-7
In a 250 ml four-neck flask, 240 g (1.368 mol)2-fluoro-3-chloronitrobenzene, temperature rise 180 ° C, accessThe dried chlorine gas,Control the temperature of the kettle at 180 ~ 190 ° C,Reaction to the way the reaction distillation 8h ~ 10h, collected distillateLiquid, washing, alkaline washing to weakly alkaline, stratified organic phase vacuum distillation in 2,6 - dichlorofluorobenzene 210g, content 99. 7%, yield 93%
References:
Binhai Yongtai Medical Chemical Co Ltd;He, Renbao;Shao, Hongming CN102249881, 2016, B Location in patent:Paragraph 0015; 0050-0051
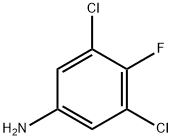
2729-34-2
121 suppliers
$5.00/250mg

2268-05-5
237 suppliers
$5.00/5g

19393-92-1
190 suppliers
$10.00/5g

2268-05-5
237 suppliers
$5.00/5g
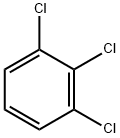
87-61-6
230 suppliers
$14.00/25g

2268-05-5
237 suppliers
$5.00/5g
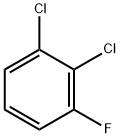
36556-50-0
115 suppliers
$10.00/1g

36556-47-5
91 suppliers
inquiry
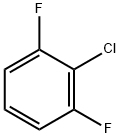
38361-37-4
190 suppliers
$20.00/5g

20098-48-0
213 suppliers
$6.00/1g

2268-05-5
237 suppliers
$5.00/5g