

*Storage: {[sel_prStorage]}
*Shipping: {[sel_prShipping]}
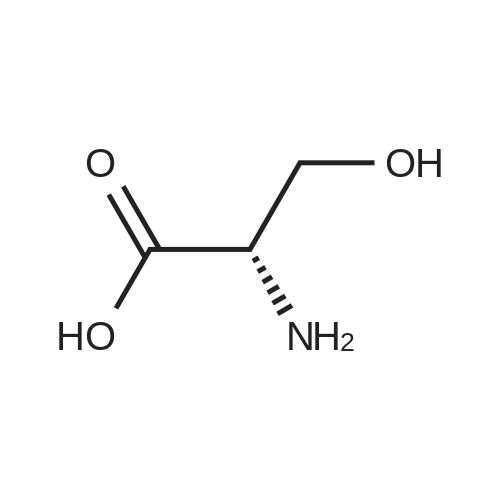
L-Serine is a non-essential amino acid that plays a critical role in cell proliferation and metabolism. It is suitable for biological research and studies on amino acid metabolism-related diseases.
Synonyms: Serine;(S)-Serine;L-Serine
4.5




 *For research use only!
*For research use only!
Change View
| Size | Price | VIP Price | USA Stock *0-1 Day |
Global Stock *5-7 Days |
In Stock | ||
| {[ item.pr_size ]} |
Inquiry
{[ getRatePrice(item.pr_usd, 1,1,item.pr_is_large_size_no_price, item.pr_usd) ]} {[ getRatePrice(item.pr_usd,item.pr_rate,1,item.pr_is_large_size_no_price, item.discount_usd) ]} {[ getRatePrice(item.pr_usd, 1,1,item.pr_is_large_size_no_price, item.pr_usd) ]} |
Inquiry {[ getRatePrice(item.pr_usd,item.pr_rate,item.mem_rate,item.pr_is_large_size_no_price, item.vip_usd) ]} | Inquiry {[ item.pr_usastock ]} In Stock Inquiry - | {[ item.pr_chinastock ]} {[ item.pr_remark ]} In Stock 1-2 weeks - Inquiry - | Login | - + | Inquiry |
Please Login or Create an Account to: See VIP prices and availability
1-2weeks
Inquiry
{[ getRatePrice(item.pr_usd,item.pr_rate,item.mem_rate,item.pr_is_large_size_no_price, item.vip_usd) ]}
{[ getRatePrice(item.pr_usd, 1,1,item.pr_is_large_size_no_price, item.pr_usd) ]}
{[ getRatePrice(item.pr_usd,1,item.mem_rate,item.pr_is_large_size_no_price, item.pr_usd) ]}
Inquiry
{[ getRatePrice(item.pr_usd,item.pr_rate,1,item.pr_is_large_size_no_price, item.vip_usd) ]}
{[ getRatePrice(item.pr_usd, 1,1,item.pr_is_large_size_no_price, item.pr_usd) ]}
{[ getRatePrice(item.pr_usd, 1,1,item.pr_is_large_size_no_price, item.pr_usd) ]}
In Stock
- +
Please Login or Create an Account to: See VIP prices and availability
Search for reports by entering the product batch number.
Batch number can be found on the product's label following the word 'Batch'.
Search for reports by entering the product batch number.
Batch number can be found on the product's label following the word 'Batch'.
Search for reports by entering the product batch number.
Batch number can be found on the product's label following the word 'Batch'.
Search for reports by entering the product batch number.
Batch number can be found on the product's label following the word 'Batch'.
Search for reports by entering the product batch number.
Batch number can be found on the product's label following the word 'Batch'.
| CAS No. : | 56-45-1 |
| Formula : | C3H7NO3 |
| Linear Structure Formula : | NH3CH(CH2OH)CO2 |
| M.W : | 105.09 |
| Synonyms : |
Serine;(S)-Serine;L-Serine
|
| MDL No. : | MFCD00064224 |
| InChI Key : | MTCFGRXMJLQNBG-REOHCLBHSA-N |
| Pubchem ID : | 5951 |
| GHS Pictogram: |

|
| Signal Word: | Warning |
| Hazard Statements: | H315-H319-H335 |
| Precautionary Statements: | P261-P305+P351+P338 |
| Num. heavy atoms | 7 |
| Num. arom. heavy atoms | 0 |
| Fraction Csp3 | 0.67 |
| Num. rotatable bonds | 2 |
| Num. H-bond acceptors | 4.0 |
| Num. H-bond donors | 3.0 |
| Molar Refractivity | 22.18 |
| TPSA ? Topological Polar Surface Area: Calculated from |
83.55 ?2 |
| Log Po/w (iLOGP)? iLOGP: in-house physics-based method implemented from |
0.18 |
| Log Po/w (XLOGP3)? XLOGP3: Atomistic and knowledge-based method calculated by |
-3.07 |
| Log Po/w (WLOGP)? WLOGP: Atomistic method implemented from |
-1.61 |
| Log Po/w (MLOGP)? MLOGP: Topological method implemented from |
-3.91 |
| Log Po/w (SILICOS-IT)? SILICOS-IT: Hybrid fragmental/topological method calculated by |
-1.45 |
| Consensus Log Po/w? Consensus Log Po/w: Average of all five predictions |
-1.97 |
| Log S (ESOL):? ESOL: Topological method implemented from |
1.57 |
| Solubility | 3950.0 mg/ml ; 37.5 mol/l |
| Class? Solubility class: Log S scale |
Highly soluble |
| Log S (Ali)? Ali: Topological method implemented from |
1.88 |
| Solubility | 7970.0 mg/ml ; 75.9 mol/l |
| Class? Solubility class: Log S scale |
Highly soluble |
| Log S (SILICOS-IT)? SILICOS-IT: Fragmental method calculated by |
1.3 |
| Solubility | 2080.0 mg/ml ; 19.8 mol/l |
| Class? Solubility class: Log S scale |
Soluble |
| GI absorption? Gatrointestinal absorption: according to the white of the BOILED-Egg |
High |
| BBB permeant? BBB permeation: according to the yolk of the BOILED-Egg |
No |
| P-gp substrate? P-glycoprotein substrate: SVM model built on 1033 molecules (training set) |
No |
| CYP1A2 inhibitor? Cytochrome P450 1A2 inhibitor: SVM model built on 9145 molecules (training set) |
No |
| CYP2C19 inhibitor? Cytochrome P450 2C19 inhibitor: SVM model built on 9272 molecules (training set) |
No |
| CYP2C9 inhibitor? Cytochrome P450 2C9 inhibitor: SVM model built on 5940 molecules (training set) |
No |
| CYP2D6 inhibitor? Cytochrome P450 2D6 inhibitor: SVM model built on 3664 molecules (training set) |
No |
| CYP3A4 inhibitor? Cytochrome P450 3A4 inhibitor: SVM model built on 7518 molecules (training set) |
No |
| Log Kp (skin permeation)? Skin permeation: QSPR model implemented from |
-9.12 cm/s |
| Lipinski? Lipinski (Pfizer) filter: implemented from |
0.0 |
| Ghose? Ghose filter: implemented from |
None |
| Veber? Veber (GSK) filter: implemented from |
0.0 |
| Egan? Egan (Pharmacia) filter: implemented from |
0.0 |
| Muegge? Muegge (Bayer) filter: implemented from |
3.0 |
| Bioavailability Score? Abbott Bioavailability Score: Probability of F > 10% in rat |
0.55 |
| PAINS? Pan Assay Interference Structures: implemented from |
0.0 alert |
| Brenk? Structural Alert: implemented from |
0.0 alert: heavy_metal |
| Leadlikeness? Leadlikeness: implemented from |
No; 1 violation:MW<1.0 |
| Synthetic accessibility? Synthetic accessibility score: from 1 (very easy) to 10 (very difficult) |
1.51 |
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.

| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 65% | Stage #1: With bromic acid; potassium bromide; sodium nitrite In water at -10 - 20℃; Stage #2: With bromic acid In water at 65℃; for 48 h; |
[1496] Step 2: methyl 2-bromo-3-hydroxypropanoate[1497] To a solution of L-serine (5.0 g, 47.6 mmol), a 48 w/wpercent bromic acid solution (13.0 mL, 109.5 mmol), and potassium bromide (20.0 g, 157.1 mmol) in water (44.0 mL) was added portionwise sodium nitrite (6.0 g, 80.9 mmol) at -10 ℃. The reaction mixture was stirred at room temperature overnight. The reaction mixture was saturated with sodium chloride and then extracted with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, filtered, and then evaporated. The residue was dissolved in methanol (50.0 mL) and then 48 w/wpercent bromic acid (0.2 mL) was added thereto. The reaction mixture was stirred at 65 ℃ for 2 days and then concentrated under reduced pressure to discard excess methanol. The resulting dark yellow residue was dissolved in dichloromethane (100.0 mL) and then washed with a saturated sodium hydrogen carbonate solution (50.0 mL) and brine. The organic layer was dried over anhydrous sodium sulfate, filtered, and then evaporated. The residue was dried under reduced pressure to give 5.7 g of the titled compound (Yield: 65percent).[1498] 1H NMR (CDCl3, 400 MHz) δ 4.36(t, 1H), 4.01-4.08(m, 1H), 3.93-3.97(m, 1H), 3.82(s, 3H), 2.56(t, 1H) |
| 65% | Stage #1: With bromic acid; potassium bromide; sodium nitrite In water at -10 - 20℃; Stage #2: at 65℃; for 48 h; |
Step 2: methyl 2-bromo-3-hydroxypropanoate To a solution of L-serine (5.0 g, 47.6 mmol), a 48 w/w percent bromic acid solution (13.0 mL, 109.5 mmol), and potassium bromide (20.0 g, 157.1 mmol) in water (44.0 mL) was added portionwise sodium nitrite (6.0 g, 80.9 mmol) at -10° C. The reaction mixture was stirred at room temperature overnight. The reaction mixture was saturated with sodium chloride and then extracted with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, filtered, and then evaporated. The residue was dissolved in methanol (50.0 mL) and then 48 w/w percent bromic acid (0.2 mL) was added thereto. The reaction mixture was stirred at 65° C. for 2 days and then concentrated under reduced pressure to discard excess methanol. The resulting dark yellow residue was dissolved in dichloromethane (100.0 mL) and then washed with a saturated sodium hydrogen carbonate solution (50.0 mL) and brine. The organic layer was dried over anhydrous sodium sulfate, filtered, and then evaporated. The residue was dried under reduced pressure to give 5.7 g of the titled compound (Yield: 65percent). 1H NMR (CDCl3, 400 MHz) δ 4.36 (t, 1H), 4.01-4.08 (m, 1H), 3.93-3.97 (m, 1H), 3.82 (s, 3H), 2.56 (t, 1H) |