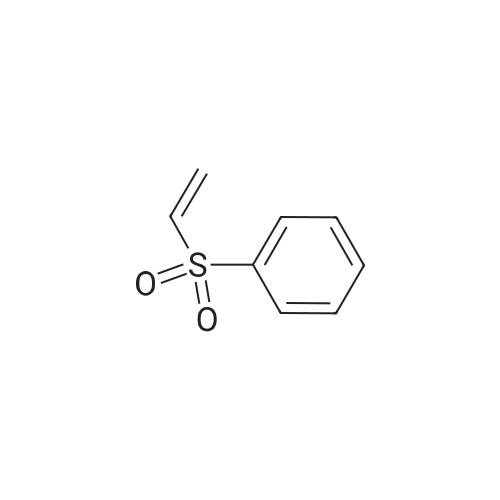


*Storage: {[sel_prStorage]}
*Shipping: {[sel_prShipping]}
4.5




 *For research use only!
*For research use only!
Change View
| Size | Price | VIP Price | USA Stock *0-1 Day |
Global Stock *5-7 Days |
In Stock | ||
| {[ item.pr_size ]} |
Inquiry
{[ getRatePrice(item.pr_usd, 1,1,item.pr_is_large_size_no_price, item.pr_usd) ]} {[ getRatePrice(item.pr_usd,item.pr_rate,1,item.pr_is_large_size_no_price, item.discount_usd) ]} {[ getRatePrice(item.pr_usd, 1,1,item.pr_is_large_size_no_price, item.pr_usd) ]} |
Inquiry {[ getRatePrice(item.pr_usd,item.pr_rate,item.mem_rate,item.pr_is_large_size_no_price, item.vip_usd) ]} | Inquiry {[ item.pr_usastock ]} In Stock Inquiry - | {[ item.pr_chinastock ]} {[ item.pr_remark ]} In Stock 1-2 weeks - Inquiry - | Login | - + | Inquiry |
Please Login or Create an Account to: See VIP prices and availability
1-2weeks
Inquiry
{[ getRatePrice(item.pr_usd,item.pr_rate,item.mem_rate,item.pr_is_large_size_no_price, item.vip_usd) ]}
{[ getRatePrice(item.pr_usd, 1,1,item.pr_is_large_size_no_price, item.pr_usd) ]}
{[ getRatePrice(item.pr_usd,1,item.mem_rate,item.pr_is_large_size_no_price, item.pr_usd) ]}
Inquiry
{[ getRatePrice(item.pr_usd,item.pr_rate,1,item.pr_is_large_size_no_price, item.vip_usd) ]}
{[ getRatePrice(item.pr_usd, 1,1,item.pr_is_large_size_no_price, item.pr_usd) ]}
{[ getRatePrice(item.pr_usd, 1,1,item.pr_is_large_size_no_price, item.pr_usd) ]}
In Stock
-
+
Please Login or Create an Account to: See VIP prices and availability
Search for reports by entering the product batch number.
Batch number can be found on the product's label following the word 'Batch'.
Search for reports by entering the product batch number.
Batch number can be found on the product's label following the word 'Batch'.
Search for reports by entering the product batch number.
Batch number can be found on the product's label following the word 'Batch'.
Search for reports by entering the product batch number.
Batch number can be found on the product's label following the word 'Batch'.
Search for reports by entering the product batch number.
Batch number can be found on the product's label following the word 'Batch'.
| CAS No. : | 5535-48-8 |
| Formula : | C8H8O2S |
| M.W : | 168.21 |
| MDL No. : | MFCD00007554 |
| InChI Key : | UJTPZISIAWDGFF-UHFFFAOYSA-N |
| Pubchem ID : | 79664 |
| GHS Pictogram: |


|
| Signal Word: | Danger |
| Hazard Statements: | H302-H315-H317-H318-H335 |
| Precautionary Statements: | P261-P280-P305+P351+P338 |
| Class: | 8 |
| UN#: | 1759 |
| Packing Group: | Ⅲ |
| Num. heavy atoms | 11 |
| Num. arom. heavy atoms | 6 |
| Fraction Csp3 | 0.0 |
| Num. rotatable bonds | 2 |
| Num. H-bond acceptors | 2.0 |
| Num. H-bond donors | 0.0 |
| Molar Refractivity | 43.87 |
| TPSA ? Topological Polar Surface Area: Calculated from |
42.52 ?2 |
| Log Po/w (iLOGP)? iLOGP: in-house physics-based method implemented from |
1.61 |
| Log Po/w (XLOGP3)? XLOGP3: Atomistic and knowledge-based method calculated by |
0.99 |
| Log Po/w (WLOGP)? WLOGP: Atomistic method implemented from |
2.68 |
| Log Po/w (MLOGP)? MLOGP: Topological method implemented from |
1.76 |
| Log Po/w (SILICOS-IT)? SILICOS-IT: Hybrid fragmental/topological method calculated by |
1.43 |
| Consensus Log Po/w? Consensus Log Po/w: Average of all five predictions |
1.7 |
| Log S (ESOL):? ESOL: Topological method implemented from |
-1.78 |
| Solubility | 2.8 mg/ml ; 0.0167 mol/l |
| Class? Solubility class: Log S scale |
Very soluble |
| Log S (Ali)? Ali: Topological method implemented from |
-1.47 |
| Solubility | 5.68 mg/ml ; 0.0338 mol/l |
| Class? Solubility class: Log S scale |
Very soluble |
| Log S (SILICOS-IT)? SILICOS-IT: Fragmental method calculated by |
-2.76 |
| Solubility | 0.292 mg/ml ; 0.00173 mol/l |
| Class? Solubility class: Log S scale |
Soluble |
| GI absorption? Gatrointestinal absorption: according to the white of the BOILED-Egg |
High |
| BBB permeant? BBB permeation: according to the yolk of the BOILED-Egg |
Yes |
| P-gp substrate? P-glycoprotein substrate: SVM model built on 1033 molecules (training set) |
No |
| CYP1A2 inhibitor? Cytochrome P450 1A2 inhibitor: SVM model built on 9145 molecules (training set) |
No |
| CYP2C19 inhibitor? Cytochrome P450 2C19 inhibitor: SVM model built on 9272 molecules (training set) |
No |
| CYP2C9 inhibitor? Cytochrome P450 2C9 inhibitor: SVM model built on 5940 molecules (training set) |
No |
| CYP2D6 inhibitor? Cytochrome P450 2D6 inhibitor: SVM model built on 3664 molecules (training set) |
No |
| CYP3A4 inhibitor? Cytochrome P450 3A4 inhibitor: SVM model built on 7518 molecules (training set) |
No |
| Log Kp (skin permeation)? Skin permeation: QSPR model implemented from |
-6.62 cm/s |
| Lipinski? Lipinski (Pfizer) filter: implemented from |
0.0 |
| Ghose? Ghose filter: implemented from |
None |
| Veber? Veber (GSK) filter: implemented from |
0.0 |
| Egan? Egan (Pharmacia) filter: implemented from |
0.0 |
| Muegge? Muegge (Bayer) filter: implemented from |
1.0 |
| Bioavailability Score? Abbott Bioavailability Score: Probability of F > 10% in rat |
0.55 |
| PAINS? Pan Assay Interference Structures: implemented from |
0.0 alert |
| Brenk? Structural Alert: implemented from |
0.0 alert: heavy_metal |
| Leadlikeness? Leadlikeness: implemented from |
No; 1 violation:MW<1.0 |
| Synthetic accessibility? Synthetic accessibility score: from 1 (very easy) to 10 (very difficult) |
2.2 |
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.

| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With potassium tert-butylate; bis[2-(diphenylphosphino)phenyl] ether; copper(l) chloride; In toluene; at 22℃; for 20h;Inert atmosphere; Sealed tube; | General procedure: CuCl (1.50 mg, 0.0150 mmol), DPEPhos (8.10 mg, 0.0150 mmol) and KOt-Bu (1.70 mg,0.0150 mmol) were added to a 8 mL vial charged with a magnetic bar. The vial was sealedwith a cap (phenolic open top cap with gray PTFE/silicone) and purged by N2 gas for 5 min.Toluene (0.8 mL) and methyl 2-aminobenzoate (1a) (46.6 L, 0.360 mmol) were added to themixture, which was allowed for 10 min. And then a solution of phenyl vinyl sulfone (2) (50.5mg, 0.300 mmol) in toluene (0.7 mL) was added to the reaction solution. After stirring atroom temperature for 5 h, THF (5 mL) was added and the reaction was allowed to cool to 0C (ice bath). After addition of KOt-Bu (101 mg, 0.900 mmol), the resulting solution wasallowed to stir for further 1 h at 0 C. After that time, the reaction was quenched with asaturated aqueous solution of NH4Cl (1 mL) and NaHCO3 (2 mL) and washed with EtOAc (7x 3 mL). The organic layers were combined, dried over MgSO4, filtered, and concentrated invacuo. The crude product was purified by silica gel column chromatography (CH2Cl2/EtOAc,8:1) to afford the desired product 4a (76.8 mg, 0.267 mmol, 89% yield) as a yellow solid. |
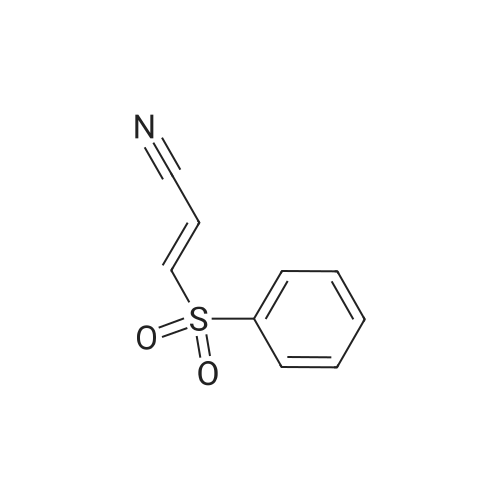
A114956[ 1424-51-7 ]
3-(Phenylsulfonyl)acrylonitrile
Similarity: 0.89
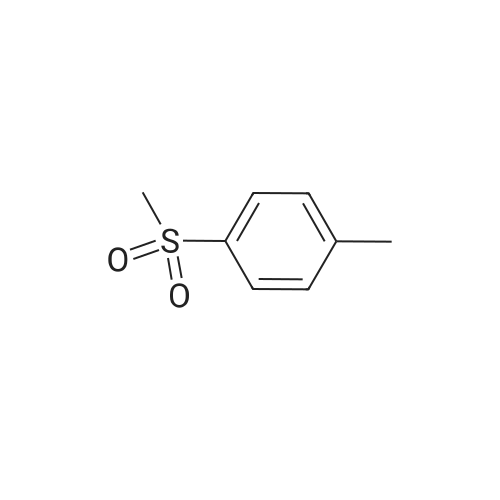
A120919[ 3185-99-7 ]
1-Methyl-4-(methylsulfonyl)benzene
Similarity: 0.87

A114956[ 1424-51-7 ]
3-(Phenylsulfonyl)acrylonitrile
Similarity: 0.89
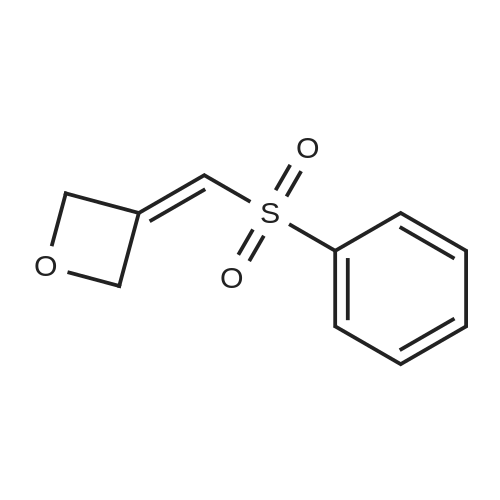
A160461[ 1221819-46-0 ]
3-((Phenylsulfonyl)methylene)oxetane
Similarity: 0.77
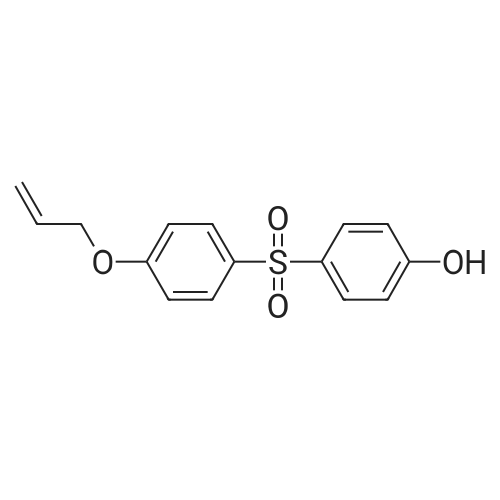
A159848[ 97042-18-7 ]
4-((4-(Allyloxy)phenyl)sulfonyl)phenol
Similarity: 0.72

A114956[ 1424-51-7 ]
3-(Phenylsulfonyl)acrylonitrile
Similarity: 0.89

A120919[ 3185-99-7 ]
1-Methyl-4-(methylsulfonyl)benzene
Similarity: 0.87