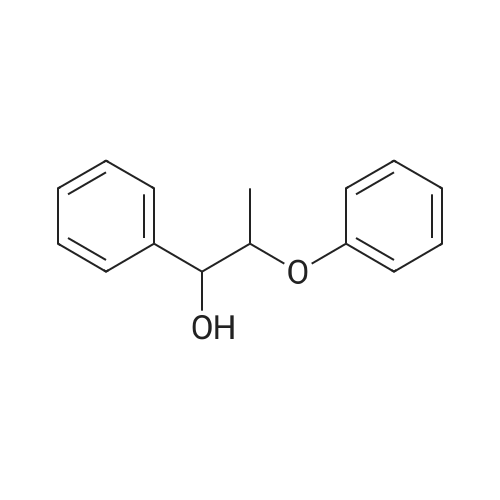
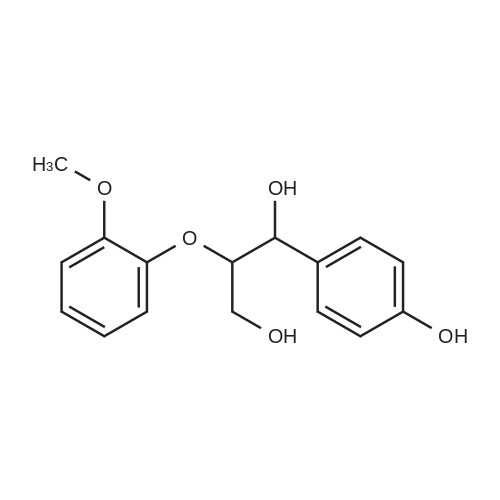
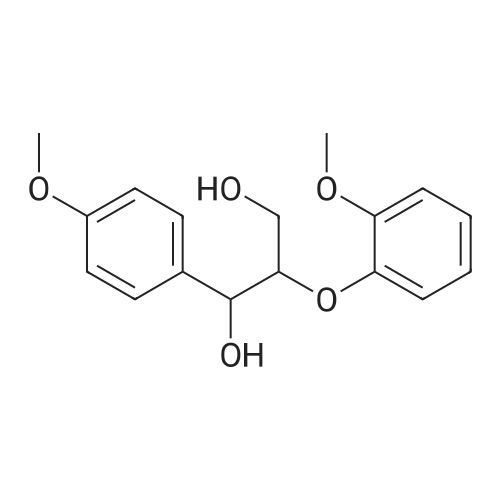
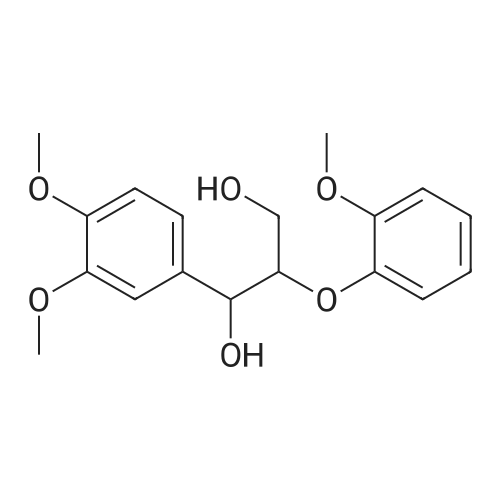
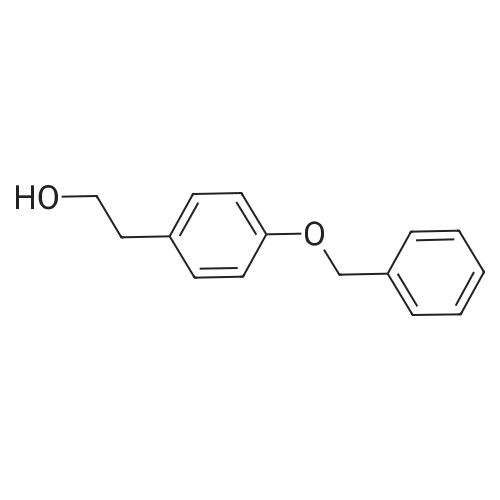
Lignin Monomer Quantification Without Standards: Using Gas Chromatography with Dual Quantitative Carbon Detection and Mass Spectrometry
Narani, Anand
;
Gao, Yu
;
Zhang, Jialiang
, et al.
Anal. Chem.,2025.
DOI:
10.1021/acs.analchem.4c06128
PubMed ID:
40033824
More
Abstract: Lignin depolymerization yields a complex mixture of monomeric products, including a wide range of highly oxygenated molecules. Quantifying these lignin monomers using existing gas chromatography (GC) with a flame ionization detector and effective carbon number methods is highly challenging due to the response variability for molecules containing heteroatoms and the inability to quantify unknown monomers. In this work, we demonstrate the potential of a GC equipped with dual detectors, a modified flame ionization detector (FID) for quantitative carbon detection (Polyarc reactor) and a mass spectrometer (GC-QCD/MS) for identifying and quantifying lignin monomers without the use of standards. Lignin depolymerization products were generated from Organosolv poplar lignin and poplar biomass through methods such as hydrogenolysis, solvolysis, and reductive catalytic fractionation. In the GC-QCD/MS, the QCD component converts all organic molecules into methane before quantification via FID, providing nearly uniform response factors for diverse compounds found within the sample, while a flow splitter directs a portion of the sample to the mass spectrometer for simultaneous molecular identification. This setup enables cost-effective, flexible, and streamlined measurements of lignin monomer carbon yields without the need for standards. Additionally, GC-QCD/MS supports the quantification of unidentified compounds within the lignin product mixture.
Purchased from AmBeed:
4249-72-3

Selective hydrodeoxygenation of oxygenated aromatic molecules using a molecular palladium catalyst covalently bound to a solid SiO2 support
Jake G. Tillou
;
Joseph J. Kuchta III
;
Nathan Thornburg
, et al.
RSC Sustain.,2024,2,2549-2558.
DOI:
10.1039/D4SU00333K
More
Abstract: The selective hydrodeoxygenation of lignin derived aromatics represents an important step towards the valorization of biomass. With this goal in mind, we synthesized a hybrid molecular/heterogeneous catalyst comprised of a (2,6-bis(1-methylbenzimidazolyl)pyridine-4′-aminopropyltrisiloxane)palladium(II) molecular catalyst covalently bound to a solid silica support through the siloxane functional group. A series of model complexes containing C–O bonds typically found in lignin biomass were explored and varying degrees of C–O bond hydrogenation were achieved. The stable covalent binding of the catalyst to the support was attributed to the observed long catalyst lifetimes which led to over 6000 catalytic turnovers without catalyst deactivation. Spectroscopic characterization of the catalyst pre- and post-catalytic reactions shows the catalyst maintains molecular integrity under the reaction conditions examined. The catalyst also exhibited complete selectivity for hydrodeoxygenation over ring hydrogenation of oxygenated aromatic molecules.
Purchased from AmBeed:
4249-72-3

Hierarchical porous activated carbon-supported ruthenium catalysts for catalytic cleavage of lignin model compounds
Pham, Xuan-Tien
;
Tran, Vy Anh
;
Tran, Lan-Trinh Thi
, et al.
Energies,2022,?15(22):8611.
DOI:
10.3390/en15228611
More
Abstract: The catalytic conversion of lignin model compounds was performed using Ru/C catalysts and an autoclave reactor. The Ru/C catalysts were prepared by the impregnation method using highly porous homemade activated carbon and characterized by XRD, SEM, and specific surface area. The catalytic reactions were performed in a high pressure/temperature reactor at different temperatures and with different solvents. The results showed that the novel Ru/C catalysts prepared from carbon supports activated by the KOH agent showed higher catalytic activity than the commercial catalyst. Ethanol and 2-propanol were suitable solvents for the cleavage of the β–O–4 ether bond of 2-phenoxy1-phenyl ethanol (~65–70% conversion) over a Ru/C-KOH-2 catalyst at 220 ?C in comparison to tert-butanol and 1-propanol solvents (~43–47% conversion of 2-phenoxy-1-phenyl ethanol). Also, the increase in reaction temperature from 200 ?C to 240 ?C enhanced the cleavage of the ether bond with an increase in phenol selectivity from 9.4% to 19.5% and improved the catalytic conversion of 2-phenoxy-1-phenyl ethanol from 46.6% to 98.5% over the Ru/C-KOH-2 catalyst and ethanol solvent. The Ru/C-KOH-2 catalyst showed outstanding conversion (98.5%) of 2-phenoxy-1-phenylethanol at 240 ?C, 1 h, ethanol solvent. This novel hierarchical porous activated carbon-supported ruthenium catalyst (Ru/C-KOH-2) can be applied for the further conversion of the lignin compound.
Keywords:
active carbon ;
biochar ;
Ru/C ;
lignin ;
β-O-4 aryl ether
Purchased from AmBeed:
4249-72-3 ;
40515-89-7

Fast screening of Depolymerized Lignin Samples Through 2D‐Liquid Chromatography Mapping
De Saegher, Tibo
;
Lauwaert, Jeroen
;
Vercammen, Joeri
, et al.
ChemistryOpen,2021,10(8):740-747.
DOI:
10.1002/open.202100088
PubMed ID:
34351071
More
Abstract: Lignin valorization and particularly its depolymerization into bio‐aromatics, has emerged as an important research topic within green chemistry. However, screening of catalysts and reaction conditions within this field is strongly constrained by the lack of analytical techniques that allow for fast and detailed mapping of the product pools. This analytical gap results from the inherent product pool complexity and the focus of the state‐of‐the‐art on monomers and dimers, overlooking the larger oligomers. In this work, this gap is bridged through the development of a quasi‐orthogonal GPC‐HPLC‐UV/VIS method that is able to separate the bio‐aromatics according to molecular weight (hydrodynamic volume) and polarity. The method is evaluated using model compounds and real lignin depolymerization samples. The resulting color plots provide a powerful graphical tool to rapidly assess differences in reaction selectivity towards monomers and dimers as well as to identify differences in the oligomers.
Purchased from AmBeed:
4249-72-3


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping