

*Storage: {[sel_prStorage]}
*Shipping: {[sel_prShipping]}
4.5




 *For research use only!
*For research use only!
Change View
| Size | Price | VIP Price | USA Stock *0-1 Day |
Global Stock *5-7 Days |
In Stock | ||
| {[ item.pr_size ]} |
Inquiry
{[ getRatePrice(item.pr_usd, 1,1,item.pr_is_large_size_no_price, item.pr_usd) ]} {[ getRatePrice(item.pr_usd,item.pr_rate,1,item.pr_is_large_size_no_price, item.discount_usd) ]} {[ getRatePrice(item.pr_usd, 1,1,item.pr_is_large_size_no_price, item.pr_usd) ]} |
Inquiry {[ getRatePrice(item.pr_usd,item.pr_rate,item.mem_rate,item.pr_is_large_size_no_price, item.vip_usd) ]} | Inquiry {[ item.pr_usastock ]} In Stock Inquiry - | {[ item.pr_chinastock ]} {[ item.pr_remark ]} In Stock 1-2 weeks - Inquiry - | Login | - + | Inquiry |
Please Login or Create an Account to: See VIP prices and availability
1-2weeks
Inquiry
{[ getRatePrice(item.pr_usd,item.pr_rate,item.mem_rate,item.pr_is_large_size_no_price, item.vip_usd) ]}
{[ getRatePrice(item.pr_usd, 1,1,item.pr_is_large_size_no_price, item.pr_usd) ]}
{[ getRatePrice(item.pr_usd,1,item.mem_rate,item.pr_is_large_size_no_price, item.pr_usd) ]}
Inquiry
{[ getRatePrice(item.pr_usd,item.pr_rate,1,item.pr_is_large_size_no_price, item.vip_usd) ]}
{[ getRatePrice(item.pr_usd, 1,1,item.pr_is_large_size_no_price, item.pr_usd) ]}
{[ getRatePrice(item.pr_usd, 1,1,item.pr_is_large_size_no_price, item.pr_usd) ]}
In Stock
- +
Please Login or Create an Account to: See VIP prices and availability
Search for reports by entering the product batch number.
Batch number can be found on the product's label following the word 'Batch'.
Search for reports by entering the product batch number.
Batch number can be found on the product's label following the word 'Batch'.
Search for reports by entering the product batch number.
Batch number can be found on the product's label following the word 'Batch'.
Search for reports by entering the product batch number.
Batch number can be found on the product's label following the word 'Batch'.
Search for reports by entering the product batch number.
Batch number can be found on the product's label following the word 'Batch'.
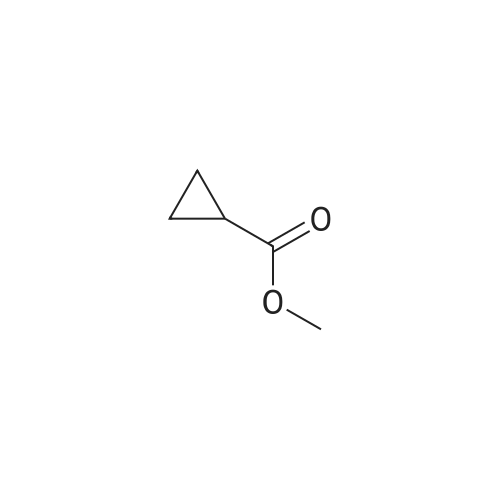
| CAS No. : | 2868-37-3 |
| Formula : | C5H8O2 |
| M.W : | 100.12 |
| MDL No. : | MFCD00001280 |
| InChI Key : | PKAHQJNJPDVTDP-UHFFFAOYSA-N |
| Pubchem ID : | 76122 |
| GHS Pictogram: |



|
| Signal Word: | Danger |
| Hazard Statements: | H225-H302-H311-H319 |
| Precautionary Statements: | P210-P270-P280-P302+P352+P312+P361+P364-P305+P351+P338+P337+P313-P370+P378 |
| Class: | 3(6.1) |
| UN#: | 1992 |
| Packing Group: | Ⅱ |
| Num. heavy atoms | 7 |
| Num. arom. heavy atoms | 0 |
| Fraction Csp3 | 0.8 |
| Num. rotatable bonds | 2 |
| Num. H-bond acceptors | 2.0 |
| Num. H-bond donors | 0.0 |
| Molar Refractivity | 25.32 |
| TPSA ? Topological Polar Surface Area: Calculated from |
26.3 ?2 |
| Log Po/w (iLOGP)? iLOGP: in-house physics-based method implemented from |
1.78 |
| Log Po/w (XLOGP3)? XLOGP3: Atomistic and knowledge-based method calculated by |
0.57 |
| Log Po/w (WLOGP)? WLOGP: Atomistic method implemented from |
0.51 |
| Log Po/w (MLOGP)? MLOGP: Topological method implemented from |
0.5 |
| Log Po/w (SILICOS-IT)? SILICOS-IT: Hybrid fragmental/topological method calculated by |
0.89 |
| Consensus Log Po/w? Consensus Log Po/w: Average of all five predictions |
0.85 |
| Log S (ESOL):? ESOL: Topological method implemented from |
-0.69 |
| Solubility | 20.5 mg/ml ; 0.205 mol/l |
| Class? Solubility class: Log S scale |
Very soluble |
| Log S (Ali)? Ali: Topological method implemented from |
-0.69 |
| Solubility | 20.2 mg/ml ; 0.202 mol/l |
| Class? Solubility class: Log S scale |
Very soluble |
| Log S (SILICOS-IT)? SILICOS-IT: Fragmental method calculated by |
-0.48 |
| Solubility | 33.4 mg/ml ; 0.333 mol/l |
| Class? Solubility class: Log S scale |
Soluble |
| GI absorption? Gatrointestinal absorption: according to the white of the BOILED-Egg |
High |
| BBB permeant? BBB permeation: according to the yolk of the BOILED-Egg |
No |
| P-gp substrate? P-glycoprotein substrate: SVM model built on 1033 molecules (training set) |
No |
| CYP1A2 inhibitor? Cytochrome P450 1A2 inhibitor: SVM model built on 9145 molecules (training set) |
No |
| CYP2C19 inhibitor? Cytochrome P450 2C19 inhibitor: SVM model built on 9272 molecules (training set) |
No |
| CYP2C9 inhibitor? Cytochrome P450 2C9 inhibitor: SVM model built on 5940 molecules (training set) |
No |
| CYP2D6 inhibitor? Cytochrome P450 2D6 inhibitor: SVM model built on 3664 molecules (training set) |
No |
| CYP3A4 inhibitor? Cytochrome P450 3A4 inhibitor: SVM model built on 7518 molecules (training set) |
No |
| Log Kp (skin permeation)? Skin permeation: QSPR model implemented from |
-6.51 cm/s |
| Lipinski? Lipinski (Pfizer) filter: implemented from |
0.0 |
| Ghose? Ghose filter: implemented from |
None |
| Veber? Veber (GSK) filter: implemented from |
0.0 |
| Egan? Egan (Pharmacia) filter: implemented from |
0.0 |
| Muegge? Muegge (Bayer) filter: implemented from |
1.0 |
| Bioavailability Score? Abbott Bioavailability Score: Probability of F > 10% in rat |
0.55 |
| PAINS? Pan Assay Interference Structures: implemented from |
0.0 alert |
| Brenk? Structural Alert: implemented from |
0.0 alert: heavy_metal |
| Leadlikeness? Leadlikeness: implemented from |
No; 1 violation:MW<1.0 |
| Synthetic accessibility? Synthetic accessibility score: from 1 (very easy) to 10 (very difficult) |
1.0 |
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.

| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| Step 1; Preparation of t-butyl 4-cyclopropylcarbonyl-2-methylcarbanilate. Under nitrogen atmosphere, to 48 ml of a t-butyl methyl ether solution containing 3.6 g of t-butyl 4-iodo-2-methylcarbanilate was added dropwise 15.1 ml of n-butyl lithium (1.6M) at -30C under stirring, and after completion of the dropwise addition, the mixture was raised to 0C and stirred for further 10 minutes. Then, this reaction mixture was cooled to -78C, 2.7 g of methylcyclopropanecarboxylate was added to the mixture, and stirring was continued at the same temperature for 4 hours, and then, at 0C for 2 hours. After completion of the reaction, 100 ml of a saturated aqueous ammonium chloride solution was added to the reaction mixture, the resulting mixture was extracted with diethyl ether (100 mlx2), the organic layer was dehydrated by saturated brine and then dried over anhydrous magnesium sulfate in this order, and the solvent was removed under reduced pressure. The residual solid was purified by silica gel column chromatography eluting with ethyl acetate-hexane (1:5) to obtain 0.93 g of the objective material as white crystals.1H NMR (CDCl3, Me4Si, 300MHz) δ 8.10 (d, J=8.7Hz, 1 H), 7.89 (dd, J=8.7, 2.1 Hz, 1H), 7.82 (d, J=1.5Hz, 1 H), 6.50 (s, 1 H), 2.6-2.7 (m, 1 H), 2.30 (s, 3H), 1.54 (s, 9H), 1.15-1.25 (m, 2H), 0.9-1.05 (m, 2H). |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 1.35 g | Example B4 A solution of methylcyclopropyl carboxylic acid (1.24 g, 12.39 mmol) and HOBt (2.466 g, 16.10 mmol) were in MeCN (31 mL) was treated portion-wise with EDC (3.09 g, 16.10 mmol), stirred at RT for 2 h, treated with NH4OH (?15M, 2.4 mL, -36 mmol) and stirred at RT overnight. The mixture was treated with 50% satd. brine, then solid NaHCO3 until saturated and extracted with EtOAc (3*). The combined organics were dried over Na2SO4 and concentrated to dryness to afford 1-methylcyclopropanecarboxamide (1.35 g, 110%) which was used without further purification. 1H NMR (400 MHz, DMSO-d6): delta 7.01 (br s, 1H), 6.81 (br s, 1H), 1.20 (s, 3H), 0.92-0.88 (m, 2H), 0.47-0.43 (m, 2H). |