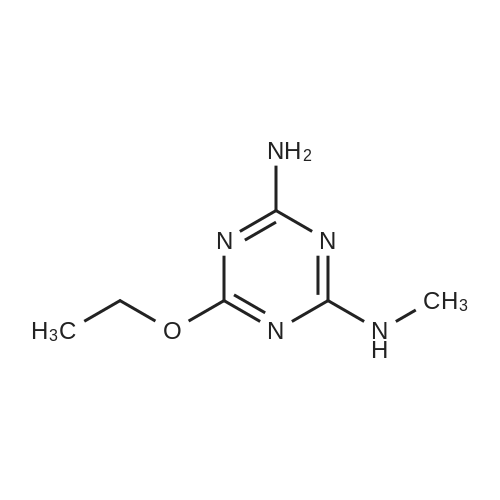
|
With hydrogenchloride; potassium tert-butylate; In ice-water; ISOPROPYLAMIDE; 2,4-dichlorophenoxyacetic acid dimethylamine; |
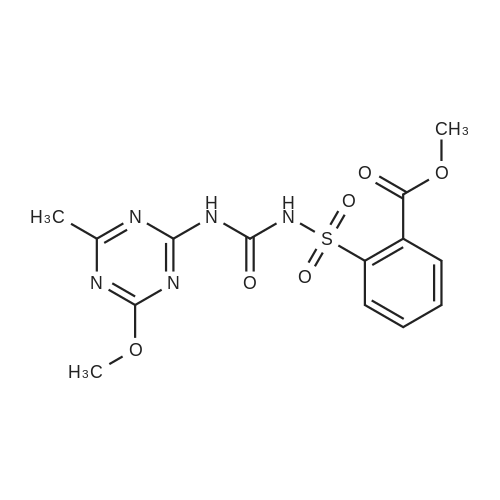
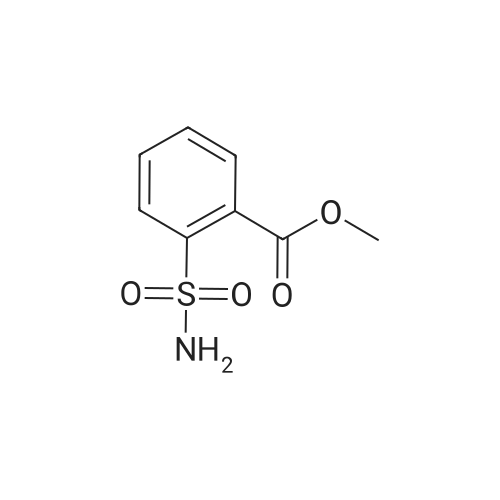
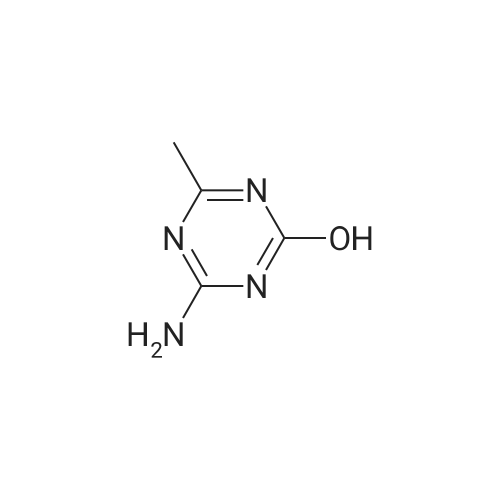
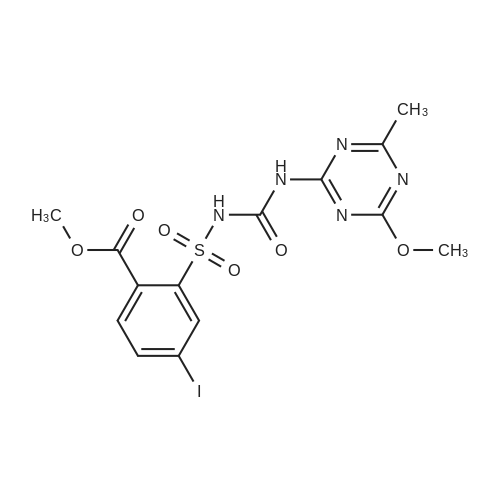
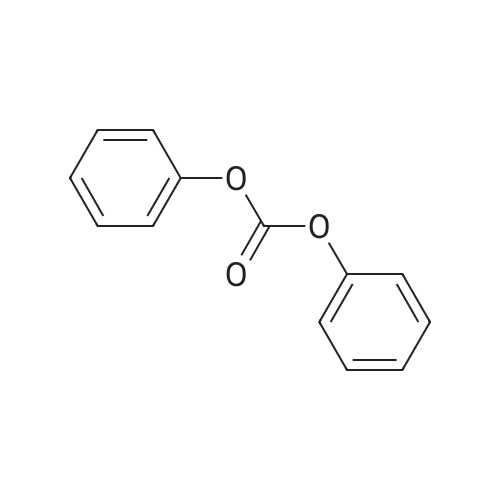
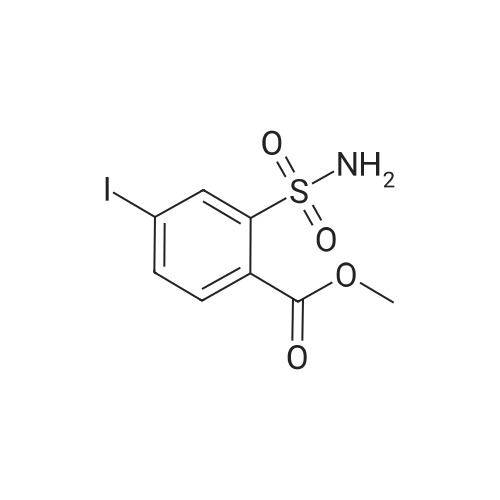
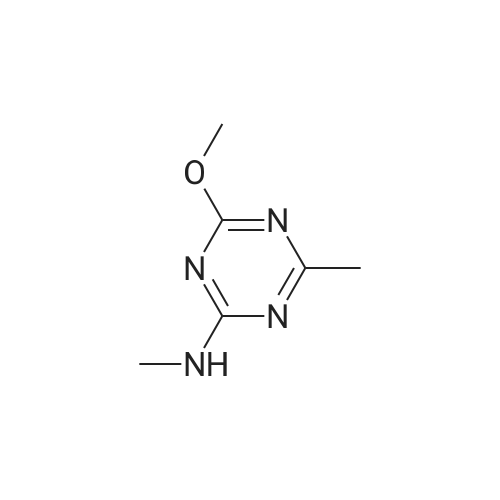
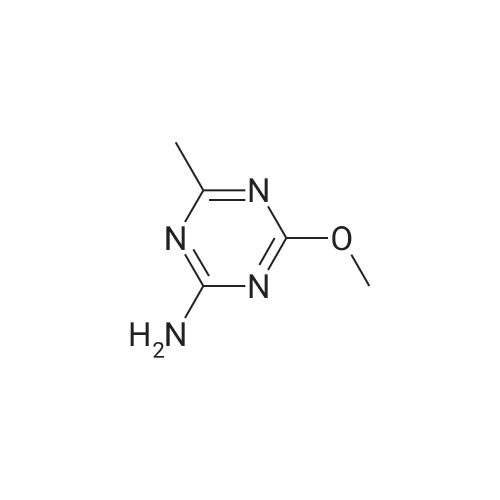
Example 2 Methyl 2-[[[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-amino]-carbonyl]-amino]-sulfonyl]-4-iodobenzoate. 2.59 g of potassium tert-butylate was added to a suspension of 2.85 g of <strong>[1668-54-8]2-amino-4-methoxy-6-methyl-1,3,5-triazine</strong> in 40 ml of dimethylacetamide (DMA) at room temperature to form a first mixture. A solution of 4.94 g of diphenyl carbonate in 20 ml of DMA was then added dropwise to the first mixture at about 5° C. to form a second mixture. The second mixture was subsequently added dropwise to a solution of 5.00 g of methyl 2-aminosulfonyl-4-iodobenzoate (92.5percent pure) in 15 ml of DMA at about 5° C., to form a third mixture. When the reaction ended, the third mixture was filtered over kieselguhr (.(R).Celite). The filtrate was introduced into a solution of 200 ml of ice-water and 10 ml of concentrated hydrochloric acid, whereby the crude urea product separated out. The crude product which separated out was then purified by stirring with methanol and diisopropyl ether and dried. The yield was 4.50 g (66percent of theory). This Example also demonstrates that the carbamate of formula (IV) can be formed and converted, without isolation, to the sulfonylurea of formula (I), in a good yield (of both (IV) and (I)), without using an alkali metal hydride or phosgene. |
|
With sodium t-butanolate; In water; 2,4-dichlorophenoxyacetic acid dimethylamine; |
Example 4 Methyl 2-[[[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)amino]carbonyl]amino]sulfonyl]-4-iodobenzoate. 5.09 g of sodium tert-butylate was added to a suspension of 3.69 g of <strong>[1668-54-8]2-amino-4-methoxy-6-methyl-1,3,5-triazine</strong> in 100 ml of DMA at room temperature. After cooling to 3-7° C., a solution of 5.64 g of diphenyl carbonate and 50 ml of DMA was added dropwise, to form a reaction mixture. The reaction mixture was then stirred at that temperature for 15 minutes. The reaction mixture was then added dropwise to a solution of 8.85 g of methyl 2-aminosulfonyl-4-iodobenzoate and 50 ml of DMA at 3-7° C., to form a resulting mixture which was stirred at 3° C. for 1 hour and at room temperature for 2 hours. The volatile components were then distilled off under reduced pressure. The residue was dissolved in 250 ml of water and acidified with concentrated hydrochloric acid (pH=2-3) whereby the crude product separated out. The crude product which separated out was washed with methanol and diisopropyl ether. After drying, 8.4 g of the desired product (purity>92percent) was obtained. This Example additionally demonstrates that the carbamate of formula (IV) can be formed and converted, without isolation, to the sulfonylurea of formula (I), with high purity, (of both (IV) and (I)) without using an alkali metal hydride or phosgene. |
|
With sodium t-butanolate; In ISOPROPYLAMIDE; 2,4-dichlorophenoxyacetic acid dimethylamine; |
Example 3 Methyl 2-[[[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-amino]-carbonyl]-amino]-sulfonyl]-4-iodobenzoate. 0.96 g of sodium tert-butylate was added to a suspension of 1.05 g of <strong>[1668-54-8]2-amino-4-methoxy-6-methyl-1,3,5-triazine</strong> in 20 ml of dimethylacetamide (DMA) at room temperature, with vigorous stirring, to form a first mixture. A solution of 1.12 g of diphenyl carbonate in 10 ml of DMA was then added to the first mixture, in the course of 7 minutes, while it was cooled in an ice bath, to form a second mixture. The second mixture was subsequently stirred for another 15 minutes while cooled in the ice bath, and a solution of 1.84 g of methyl 2-aminosulfonyl-4-iodobenzoate (92.5percent pure) in DMA was then added dropwise in the course of 7 minutes. When the reaction ended, the product was worked up as described in Example 1. 1.47 g of the desired product (58percent of theory) was thus obtained. This Example further demonstrates that the carbamate of formula (IV) can be formed and converted, without isolation, to the sulfonylurea of formula (I), in a good yield (of both (IV) and (I)), without using an alkali metal hydride or phosgene. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping