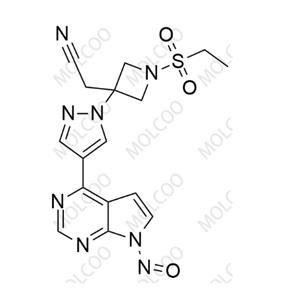
Baricitinib Impurity 64 NEW
| Price | Get Latest Price | ||
| Package | 5mg | 20mg | 50mg |
| Min. Order: | 1mg |
| Supply Ability: | 10000 |
| Update Time: | 2025-05-27 |
Product Details
| Product Name: Baricitinib Impurity 64 | Min. Order: 1mg |
| Purity: >95% HPLC | Supply Ability: 10000 |
| Release date: 2025/05/27 | |
| Molecular Formula:: C16H16N8O3S | Molecular Weight:: 400.42 |
| Appearance: Yellow soild | Storage: 2-8°C Refrigerator |
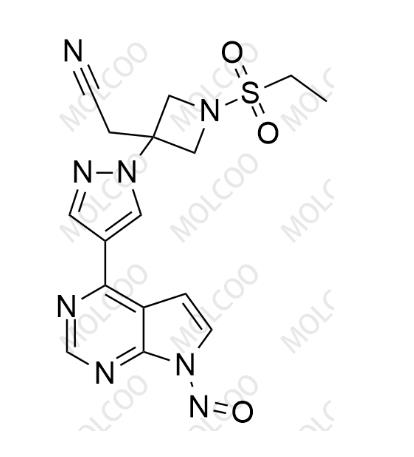
| Product Catalog: | B026064 |
| CAS No.: | |
| Product Name: | Baricitinib Impurity 64 |
| Purity: | >95% HPLC |
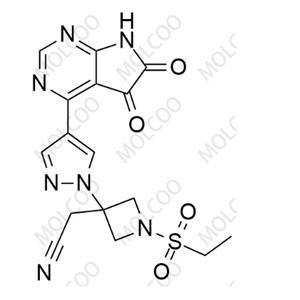
| Synonyms: | Baricitinib Nitroso Impurity 64; 2-(1-(ethylsulfonyl)-3-(4-(7-nitroso-7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)azetidin-3-yl)acetonitrile |
| Molecular Formula: | C16H16N8O3S |
| Mol. Weight: | 400.42 |
| Appearance: | Yellow soild |
| Storage: | 2-8°C Refrigerator |
| Contact: | WhatsAPP: +86 17320513646 E-mail: anna@molcoo.com |
| Note: | We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS. This product is intended for laboratory use only! |
| Background: | Current research on baricitinib nitroso impurity mainly focuses on detection and quantification methods. High - performance liquid chromatography - tandem mass spectrometry (HPLC - MS/MS) and other advanced analytical techniques are being used to accurately measure the content of this impurity in baricitinib samples. At the same time, studies are being carried out to investigate the factors that affect the formation and stability of the impurity. This includes exploring the impact of different reaction conditions in the synthesis process and the effect of storage temperature, humidity, and light on impurity formation. Pharmaceutical companies and regulatory agencies are working together to establish appropriate control strategies and acceptance criteria to ensure that the level of baricitinib nitroso impurity is within a safe range. |
Company Profile Introduction
1. Drug standards and drug impurity reference substances: provide more than 20,000 kinds of spot impurity reference substances, with sufficient supply and same-day delivery. We have a professional R&D team and comprehensive quality control testing to ensure product quality and reliability.
2. Customized synthesis of impurities and new molecules: Quickly respond to the customized needs of CDE impurities, stably supply impurities that have established quality standards, and provide customized synthesis services for new compounds in the research and development of innovative drugs.
3. Preparation and separation of unknown impurities: With a professional impurity preparation and separation technical team and SFC preparation and separation instruments, we can solve the problems of impurity preparation in complex projects.
4. Process development of new drug intermediates: provide the supply of new drugs and new molecular impurities, process optimization services, as well as the screening and impurity analysis of commercialization routes.
5. Peptide synthesis: Provide customized peptide synthesis services, and cover all kinds of degradation impurities and process impurities generated in the research and development of peptide drugs. At the same time, it provides comprehensive structural confirmation data and customized testing services.
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-24 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-03-31 | |
| $2.00/1kg |
VIP2Y
|
Jinan Million Pharmaceutical Co., Ltd
|
2023-08-11 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-03-31 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-08-06 |
INQUIRY







 China
China