| Identification | Back Directory | [Name]
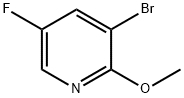
3-BROMO-5-FLUORO-2-METHOXYPYRIDINE | [CAS]
884494-81-9 | [Synonyms]
uoro-2-methoxypyridine
3-BROMO-5-FLUORO-2-METHOXYPYRIDINE
Pyridine, 3-bromo-5-fluoro-2-methoxy-
3-Bromo-5-fluoro-2-methoxypyridine 98%
3-Bromo-5-fluoro-2-methoxypyridine ,98%
3-Bromo-5-fluoropyridin-2-yl methyl ether
3-BROMO-5-FLUORO-2-METHOXYPYRIDINE ISO 9001:2015 REACH | [Molecular Formula]
C6H5BrFNO | [MDL Number]
MFCD04972397 | [MOL File]
884494-81-9.mol | [Molecular Weight]
206.01 |
| Chemical Properties | Back Directory | [Boiling point ]
188.4±35.0 °C(Predicted) | [density ]
1.621±0.06 g/cm3(Predicted) | [refractive index ]
1.5390 to 1.5430 | [storage temp. ]
Inert atmosphere,2-8°C | [form ]
Liquid | [pka]
-1.38±0.20(Predicted) | [Appearance]
Colorless to light yellow Solid-Liquid Mixture | [Sensitive ]
Light Sensitive | [CAS DataBase Reference]
884494-81-9 |
| Hazard Information | Back Directory | [Uses]
3-Bromo-5-fluoro-2-methoxypyridine is a compound used in the synthesis of 5-Fluoro-2-methoxy-3-(2R)-2-pyrrolidinylpyridine (1213093-30-1). | [Synthesis]
General steps:
1. Prepare raw material: (R)-5-fluoro-2-methoxy-3-(pyrrolidin-2-yl)pyridine.
2. Step A: Synthesis of 3-bromo-5-fluoro-2-methoxypyridine.
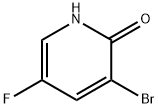
a. Add 3-bromo-5-fluoropyridin-2(1H)-one (10.0 g, 52.1 mmol) and Ag2CO3 (10.0 g, 36.5 mmol) to toluene (100 mL).
b. Slowly add iodomethane (3.89 mL, 62.5 mmol).
c. The reaction mixture was stirred at room temperature overnight.
d. Upon completion of the reaction, filter through diatomaceous earth and wash the solids with toluene.
e. The filtrate was concentrated to give the crude product.
f. The crude product was purified by silica gel column chromatography (eluent: 5-25% EtOAc/hexane) to afford 3-bromo-5-fluoro-2-methoxypyridine (4.70 g, 43.8% yield) as a colorless oil. | [References]
[1] Patent: WO2011/6074, 2011, A1. Location in patent: Page/Page column 74-75 |
|
|