| Identification | Back Directory | [Name]
(2S)-2,6-DIAMINO-N-[(1S)-1-METHYL-2-PHENYLETHYL]HEXANAMIDE DIMETHANESULFONATE | [CAS]
608137-33-3 | [Synonyms]
Ldx
Spd489
Nrp 104
Nrp-104
Spd 489
Lisdexamfetamine mesilate
Lisdexamfetamine mesylate
Lisdexamfetamine dimesylate
Lisdexamphetamine-Dimesylate
Lisdexamfetamine dimesylate solution
Lisdexamfetamine (mesylate) (exempt preparation)
(2S)-2,6-diamino-N-[(2S)-1-phenylpropan-2-yl]hexanamide,methanesulfonic acid
(2S)-2,6-DIAMINO-N-[(1S)-1-METHYL-2-PHENYLETHYL]HEXANAMIDE DIMETHANESULFONATE
(2S)-2,6-DIAMINO-N-[(1S)-1-METHYL-2-PHENYLETHYL]HEXANAMIDE DIMETHANESULFONATE USP/EP/BP | [EINECS(EC#)]
200-659-6 | [Molecular Formula]
C17H33N3O7S2 | [MDL Number]
MFCD16628114 | [MOL File]
608137-33-3.mol | [Molecular Weight]
455.59 |
| Hazard Information | Back Directory | [Description]
ADHD is a neurobehavioral disorder characterized by varying degrees of
inattention, hyperactivity, and impulsivity. ADHD is typically diagnosed in
childhood and affects 7–12% of the pediatric population in the United States with
the condition often enduring into adulthood.
While the precise mechanism of action of lisdexamfetamine
in treating ADHD is not known, amphetamines are believed to inhibit the
reuptake of the neurotransmitters dopamine and noradrenaline (norepinephrine),
thereby increasing their presynaptic availability and release into extraneuronal space. The prodrug is constructed by the condensation of D-amphetamine with the activated ester (N-hydroxysuccinimide) of bis-tert-butoxycarbonylprotected L-lysine. Lisdexamfetamine is ultimately generated by treatment with hydrochloric acid in dioxane.
The most common adverse events, comparable to other amphetamine formulations, were decreased appetite, insomnia, upper abdominal pain, and irritability. Lisdexamfetamine is contraindicated in patients with advanced arteriosclerosis, symptomatic cardiovascular disease, moderate-to-severe hypertension, hyperthyroidism, known hypersensitivity to the sympathomimetic amines, glaucoma, a predisposition to agitated states, and a history of drug abuse. In addition, the drug should not be administered during or within 14 days of treatment with monoamine oxidase inhibitors. It has also been noted that psychostimulants may exacerbate symptoms of pre-existing psychotic disorders, so caution and close observation are recommended in this patient population. | [Originator]
New River Pharmaceuticals (US) | [Uses]
Treatment of atten tion deficit hyperactivity disorder (ADHD). | [Brand name]
Vyvanse | [Synthesis]
The straightforward synthesis of lisdexamfetamine mesilate
was initiated by adding a solution of D-amphetamine (66) to
a solution of Boc-L-Lys(Boc)-OSu (65), N-methylmorpholine
and 1,4-dioxane. The resulting
mixture was partitioned between isopropyl acetate and an
acetic acid/brine solution, and the organic layer was washed
with aqueous sodium bicarbonate to give Boc-L-Lys(Boc)-
D-amphetamine (67) in 91% yield. The two primary amine
groups were liberated by reacting a solution of 67 in 1,4-
dioxane with methanesulfonic acid providing lisdexamfetamine
mesilate (X) in 92% yield.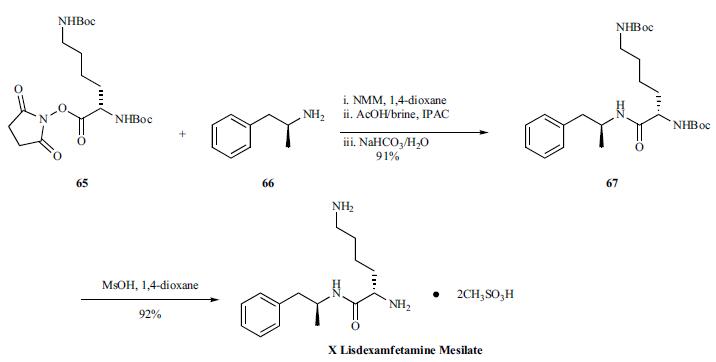
|
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
| Company Name: |
T&W GROUP
|
| Tel: |
021-61551611 13296011611 |
| Website: |
www.trustwe.com/ |
|