| Identification | Back Directory | [Name]
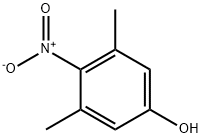
3,5-Dimethyl-4-nitrophenol | [CAS]
5344-97-8 | [Synonyms]
3,5-Dimethyl-4-nitrophenol
Phenol, 3,5-diMethyl-4-nitro- | [Molecular Formula]
C8H9NO3 | [MDL Number]
MFCD00031365 | [MOL File]
5344-97-8.mol | [Molecular Weight]
167.16 |
| Chemical Properties | Back Directory | [Melting point ]
107-108 °C(Solv: hydrochloric acid (7647-01-0)) | [Boiling point ]
311.8±30.0 °C(Predicted) | [density ]
1.263±0.06 g/cm3(Predicted) | [storage temp. ]
Inert atmosphere,2-8°C | [solubility ]
soluble in Dichloromethane, Methanol | [pka]
pK1:8.245 (25°C) | [Appearance]
Light yellow to yellow Solid | [InChI]
InChI=1S/C8H9NO3/c1-5-3-7(10)4-6(2)8(5)9(11)12/h3-4,10H,1-2H3 | [InChIKey]
MMLPWOHLKRXZJA-UHFFFAOYSA-N | [SMILES]
C1(O)=CC(C)=C([N+]([O-])=O)C(C)=C1 |
| Hazard Information | Back Directory | [Uses]
3,5-Dimethyl-4-nitrosophenol is an intermediate in the synthesis of 4-Hydroxy Xylazine which is a major metabolite of Xylazine (X748000). | [Synthesis]
The general procedure for the synthesis of 3,5-dimethyl-4-nitrophenol from 3,5-dimethylphenol was as follows: 3,5-dimethylphenol (50 g) was dissolved in 200 mL of methanesulfonic acid (MES03H) and the solution was cooled to 0 °C. One equivalent (34.8 g) of sodium nitrate (NaNO3) was added in batches at 0°C. After 18 hours of reaction, the reaction mixture was poured into 4 L of ice water under vigorous stirring. The upper aqueous phase was decanted. The residue was dissolved in 400 mL of ethyl acetate (EtOAc). The ethyl acetate extract was washed with saturated aqueous sodium bicarbonate (NaHCO3) (2 x 200 mL) and subsequently dried with saturated saline and anhydrous sodium sulfate (Na2SO4). Finally, purification was carried out on a silica gel column using a solvent mixture of ethyl acetate and n-heptane (4:1). The intermediate 3,5-dimethyl-4-nitrophenol 7.86 g was obtained in 11% yield. | [References]
[1] Patent: US6337423, 2002, B1. Location in patent: Example 4, 22
[2] Patent: WO2005/28479, 2005, A2. Location in patent: Page/Page column 54-55
[3] Journal of the American Chemical Society, 1977, vol. 99, # 15, p. 5146 - 5151
[4] Patent: US6462034, 2002, B1 |
|
|