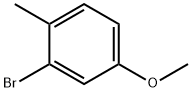
| Identification | Back Directory | [Name]
2-BroMo-4-Methoxy-1-Methyl-benzene | [CAS]
36942-56-0 | [Synonyms]
3-BroMo-4-Methylanisole
2-Bromo-4-methoxytoluene
4-Methoxy-2-Bromo toluene
Benzene, 2-broMo-4-Methoxy-1-Methyl-
3-Bromo-4-methylanisole 2-Bromo-4-methoxytoluene | [Molecular Formula]
C8H9BrO | [MDL Number]
MFCD12547797 | [MOL File]
36942-56-0.mol | [Molecular Weight]
201.06 |
| Chemical Properties | Back Directory | [Melting point ]
144-145.5℃ | [Boiling point ]
224.7±20.0℃ (760 Torr) | [density ]
1.378±0.06 g/cm3 (20 ºC 760 Torr) | [Fp ]
101.8±7.8℃ | [storage temp. ]
Sealed in dry,Room Temperature | [Appearance]
Colorless to light yellow Liquid |
| Hazard Information | Back Directory | [Chemical Properties]
Pale yellow solid | [Synthesis]
5-Methoxy-2-methylaniline (1.3 g, 9.48 mmol) was used as a raw material in a mixed solution of 48% hydrobromic acid (13 mL) and ethanol (10 mL), and cooled to 0°C. At this temperature, an aqueous solution (5 mL) of sodium nitrite (0.78 g, 11.30 mmol) was slowly added, and the dropwise addition process was controlled to be completed within 15 min and the reaction temperature was kept between 0-5 °C. After the reaction solution was kept at 0 °C and stirred for 1 h, it was transferred to a preheated to boiling solution of cuprous bromide (6.8 g, 47.4 mmol) in 48% hydrobromic acid (25 mL). The mixed solution was refluxed overnight. After the reaction was complete, it was cooled to room temperature and the reaction mixture was diluted with water (50 mL). The acidic aqueous phase was extracted with ethyl acetate (2 x 50 mL). The organic phases were combined, washed once with saturated sodium chloride solution (50 mL) and dried over anhydrous sodium sulfate. The desiccant was removed by filtration and the solvent was removed by concentration under reduced pressure. The crude product was purified by chromatography on a silica gel column (3.5 x 16 cm), using hexane as eluent, to afford the colorless oily target product 3-bromo-4-methylanisole (750 mg, 39% yield). | [References]
[1] Patent: US2005/245524, 2005, A1. Location in patent: Page/Page column 98
[2] Acta Chemica Scandinavica (1947-1973), 1952, vol. 6, p. 1137,1400
[3] Anales de la Real Sociedad Espanola de Fisica y Quimica, 1959, vol. 55, p. 683,689
[4] Analytical Chemistry, 1992, vol. 64, # 8, p. 837 - 842
[5] Patent: WO2006/99735, 2006, A1. Location in patent: Page/Page column 33 |
|
|