| Identification | Back Directory | [Name]
[(Difluoromethyl)sulfonyl]benzene | [CAS]
1535-65-5 | [Synonyms]
PhSO2CF2H
Phenyl difluoromethyl sulfone
Difluoromethyl phenyl sulfone
Difluoromethylphenylsulfone,95%
[(Difluoromethyl)sulfonyl]benzene
Difluoromethyl Phenyl Sulfone >
1-(difluoroMethylsulfonyl)benzene
Difluoromethyl phenyl sulfone >=97%
Benzene, [(difluoroMethyl)sulfonyl]- | [Molecular Formula]
C7H6F2O2S | [MDL Number]
MFCD01050170 | [MOL File]
1535-65-5.mol | [Molecular Weight]
192.18 |
| Chemical Properties | Back Directory | [Melting point ]
24-25℃ | [Boiling point ]
115-120 °C(Press: 7 Torr) | [density ]
1.348 | [Fp ]
128℃ | [refractive index ]
1.5000 | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
liquid | [color ]
colorless | [Water Solubility ]
Soluble in chloroform and water. | [BRN ]
2259218 |
| Hazard Information | Back Directory | [Description]
Difluoromethyl phenyl sulfone is a powerful nucleophilic difluoromethylation reagent due to the
high reactivity of the sulfonyl-stabilized difluoromethyl anion towards many electrophiles
including carbonyls, imines, alkyl halides, and cyclic sulfates and sulfamidates. In the nucleophilic
reaction step, depending on the substrate structure, strong bases are used to generate the
nucleophilic (phenylsulfonyl)difluoromethyl anion in situ. In the desulfonylation step,
sodium/mercury amalgam and magnesium are the commonly used reductive reagents. Besides, the
(phenylsulfonyl)difluoromethylated compounds can undergo β-elimination to afford
gem-difluoroalkenes. | [Chemical Properties]
light yellow liquid | [Uses]
Efficient reagent for difluoromethylation of carbonyls and aldehydes. | [Reactions]
(1) Difluoromethylation of alkyl halides.
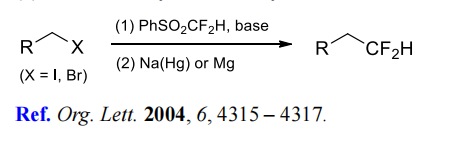
(2) Difluoromethylation of aldehydes and ketones.

(3) Difluoromethylenation of aldimines and ketimines.

(4) Difluoromethylation of cyclic sulfates and sulfamidates.

(5) (Phenylsulfonyl)difluoromethylation of carboxylic acid esters.

(6) Difluoromethylenation of alkyl halides.

(7) Difluoromethylenation of aromatic aldehydes.

| [References]
[1] G. PRAKASH. Nucleophilic difluoromethylation of primary alkyl halides using difluoromethyl phenyl sulfone as a difluoromethyl anion equivalent.[J]. Organic Letters, 2004. DOI:10.1002/CHIN.200509059.
[2] G. K. SURYA PRAKASH. Difluoromethyl Phenyl Sulfone, a Difluoromethylidene Equivalent: Use in the Synthesis of 1,1-Difluoro-1-alkenes.[J]. ChemInform, 2005. DOI:10.1002/chin.200504098.
[3] G. K. SURYA PRAKASH. Nucleophilic Difluoromethylation of Primary Alkyl Halides Using Difluoromethyl Phenyl Sulfone as a Difluoromethyl Anion Equivalent[J]. Organic Letters, 2004, 6 23: 4315-4317. DOI:10.1021/ol048166i.
|
|
|